- Wolfson School of Medicine, University of Glasgow, Scotland, United Kingdom,
- Department of Neurosurgery, Allama Iqbal Medical College, Jinnah Hospital, Lahore, Pakistan,
- School of Medicine, King Edward Medical University, Lahore, Pakistan,
- Department of Oncology, Allama Iqbal Medical College, Jinnah Hospital, Lahore, Pakistan.
Correspondence Address:
Mohammad Ashraf, Wolfson School of Medicine, University of Glasgow, Scotland, United Kingdom.
DOI:10.25259/SNI_897_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohammad Ashraf1, Ghulam Naseeruddin2, Shah Gul Zahra3, Kashif Ali Sultan2, Usman Ahmad Kamboh2, Mehwish Manzoor4, Minaam Farooq3, Manzoor Ahmad2, Naveed Ashraf2. Intracerebral hemorrhage as the first symptomatic manifestation of chronic myeloid leukemia (chronic phase): A case report and literature review. 06-Jan-2023;14:5
How to cite this URL: Mohammad Ashraf1, Ghulam Naseeruddin2, Shah Gul Zahra3, Kashif Ali Sultan2, Usman Ahmad Kamboh2, Mehwish Manzoor4, Minaam Farooq3, Manzoor Ahmad2, Naveed Ashraf2. Intracerebral hemorrhage as the first symptomatic manifestation of chronic myeloid leukemia (chronic phase): A case report and literature review. 06-Jan-2023;14:5. Available from: https://surgicalneurologyint.com/surgicalint-articles/12094/
Abstract
Background: Chronic myeloid leukemia (CML) is mostly asymptomatic at diagnosis. Intracerebral hemorrhage (ICH), as the first presentation of CML in its chronic phase (CP) has only once been reported in the literature. In addition, CML (CP) patients developing ICH are equally rare, with only eight cases reported. ICH is more commonly associated with CML progressing to its end stage (accelerated phase [AP] and blast crisis [BC]). The pathophysiology of ICH in CML-CP is postulated to be due to leukostasis, unlike in the CML-AP/BC, where thrombocytopenia and coagulopathy are the underlying mechanisms. This case adds to the scarce literature on a rare and challenging complication of ICH in CML-CP, especially as these patients tend to rebleed and management is uncertain.
Case Description: A 22-year-old male presented with a 2-week history of headaches and vomiting, associated with a 1-week history of the left-sided weakness. Initial blood work revealed hyperleukocytosis. The patient was investigated for CML with intracranial involvement. During his stay, his Glasgow coma score (GCS) dropped (from 14 to 11), prompting an urgent CT scan which revealed a large resolving ICH with perifocal edema and midline shift. A decompressive hemicraniectomy with expansion duraplasty was performed to alleviate the mass effect and reduce intracranial pressure. Three hours postoperatively, the patient developed an extradural hematoma which needed prompt evacuation. A postoperative CT revealed an improved midline shift, and after 7 days, his GCS improved to 15, and he began oncological treatment. Neurological symptoms were experienced by our patient at presentation with hyperleukocytosis on full blood count, which may implicate leukostasis as an underlying mechanism.
Conclusion: Even in the CP, CML patients presenting with mild neurological symptoms should be investigated to exclude intracranial bleeds. As these patients tend to rebleed, they should be conservatively managed unless there is a need to alleviate intracranial pressure.
Keywords: Chronic myeloid leukemia, Chronic phase, Hyperleukocytosis, Intracerebral hemorrhage, Intracranial involvement, Leukostasis
INTRODUCTION
Chronic myeloid leukemia (CML) is a myeloproliferative disorder where there is neoplastic proliferation of mature myeloid lineage cells.[
There are three stages of CML. The chronic phase (CML-CP) represents 90–95% of all patients, the symptoms of which are due to bone marrow/hematological abnormalities.[
Patients with CML-CP may evolve to the accelerated phase (CML-AP) and eventually progress to blast crisis (CML-BC) which presents as acute leukemia and worsening of fundamental symptoms of bleeding, fever, and infections as the blasts cells predominate and crowd the bone marrow impairing normal hematopoiesis leading to pancytopenias.[
Solid brain tumors are commonly known to manifest as intracerebral hemorrhages (ICHs).[
CASE PRESENTATION
A 22-year-old male presented to medical acute receiving on July 15, 2022, with a 2-week history of intermittent headaches and vomiting. In addition, there was a 1-week history of the left-sided weakness and a 3-day history of altered sensorium. His initial Glasgow coma score (GCS) was 14 (E4, V4, M6), and his pupils were bilaterally reactive and normal in size. Neurological examination revealed weakness with left-sided power of grade 2 in his arm and leg (MRC scale). Babinski was upgoing. Marked splenomegaly was present on palpation of the abdomen and ultrasound spleen. His initial blood count revealed leukocytosis, and his total leukocyte count was 447 × 10^9/L (reference range 4–11 × 10^9/L). This raised suspicion of hematological malignancy. A full blood count (FBC) with peripheral smear showed 38% myelocytes, 7% metamyelocytes, and 1% blast cells, making CML the main provisional diagnosis with an intracerebral complication.
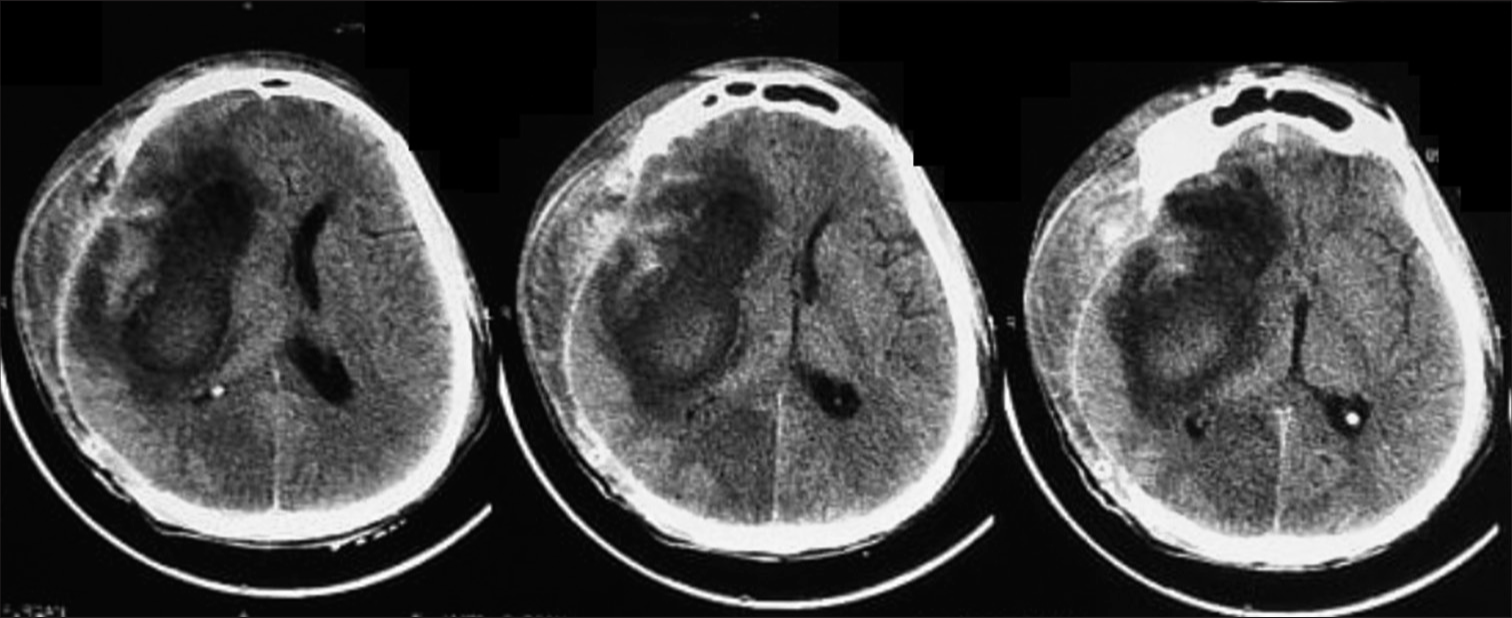
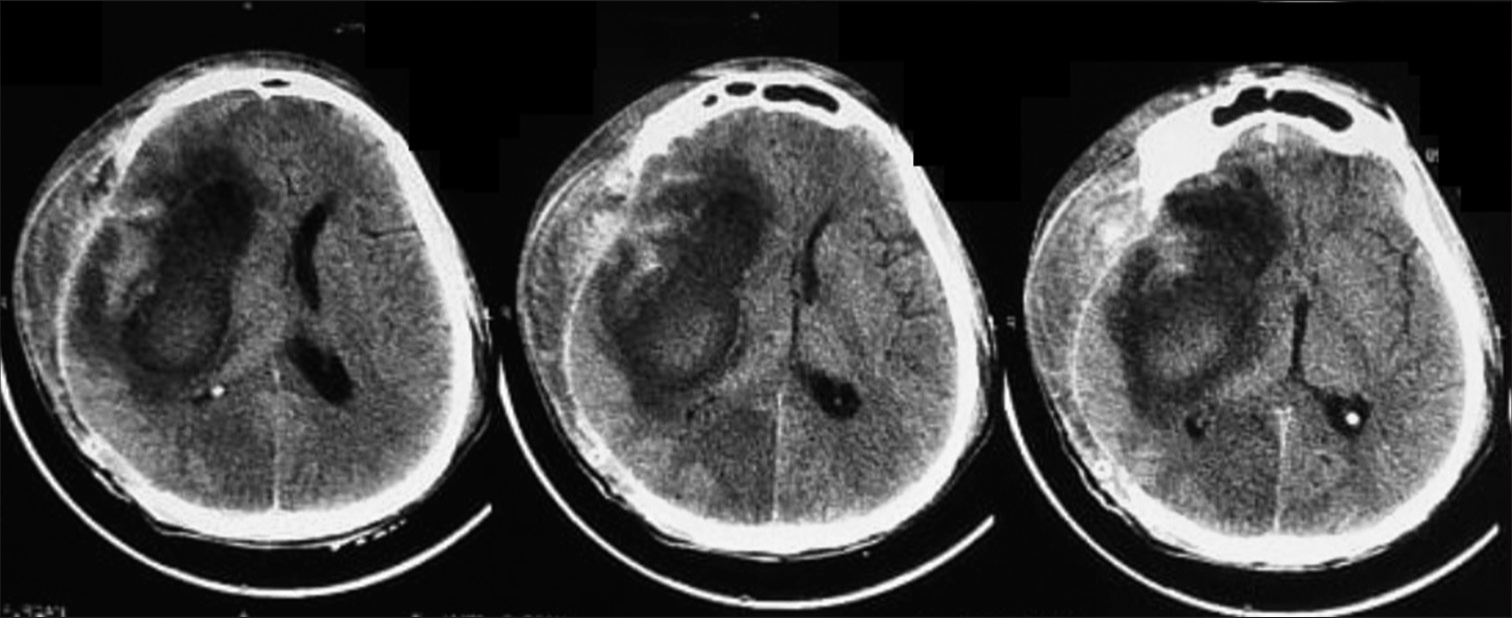
A CT scan and an urgent bone marrow biopsy were done the next day. During this time, the patient’s conscious level acutely deteriorated with a GCS of 11 (E3, V2, M6). He was administered mannitol 200 mL IV bolus and was referred to our neurosurgery department. The CT scan revealed a right frontal-temporal-parietal intracerebral hematoma with perifocal edema and a midline shift of 2.5 cm [
His remaining investigations performed by the medical admissions team, as advised by hematology/oncology, before he was shifted to neurosurgery, revealed as follows. Platelet count was 202 × 10^9/L (reference range 150–300 × 10^9/L). A peripheral smear showing red blood cell morphology included anisocytosis with poikilocytosis and macrocytosis with a few teardrop cells. Differential leukocyte count confirmed that there were 50% neutrophils, 38% myelocyte, 7% metamyelocyte, 2% basophil, 1% lymphocyte, and 1% blast cells. Bone marrow aspirate taken from the posterior iliac crest showed hypercellular smears with reduced erythropoiesis and increased leucopoiesis with all stages of maturation seen. The myeloid-to-erythroid precursor ratio was 25:1. Megakaryocytes were present mainly hypolobulated, and blasts constituted 1% of nucleated marrow cells. CML in the CP was confirmed as blast cells in the bone marrow and peripheral blood were <10%.[
During his stay in the neurosurgical ward and transfer to our oncology colleagues, cytogenetics was performed, including fluorescence in situ hybridisation, and BCR-ABL t(9;22) (q34;q11.2). A BCR-ABL fusion confirmed the diagnosis of CML, as this translocation was detected in 82.5% of cells. The patient is now commenced on a tyrosine kinase inhibitor imatinib by our oncology colleagues with risk stratification based on the Sokal score.
DISCUSSION
In our case, the patient did not have any history constitutional CML-CP symptoms, nor did they have any risk factors for ICH such as smoking, diabetes, hypertension, atrial fibrillation, and history of head trauma, and the patient did not drink alcohol. ICH is more prevalent in patients with AML and CML-BC than with CML-CP.[
The main cause of ICH in CML-BC is thrombocytopenia, but hyperleukocytosis is suggested as the main etiology of ICH in CML-CP.[
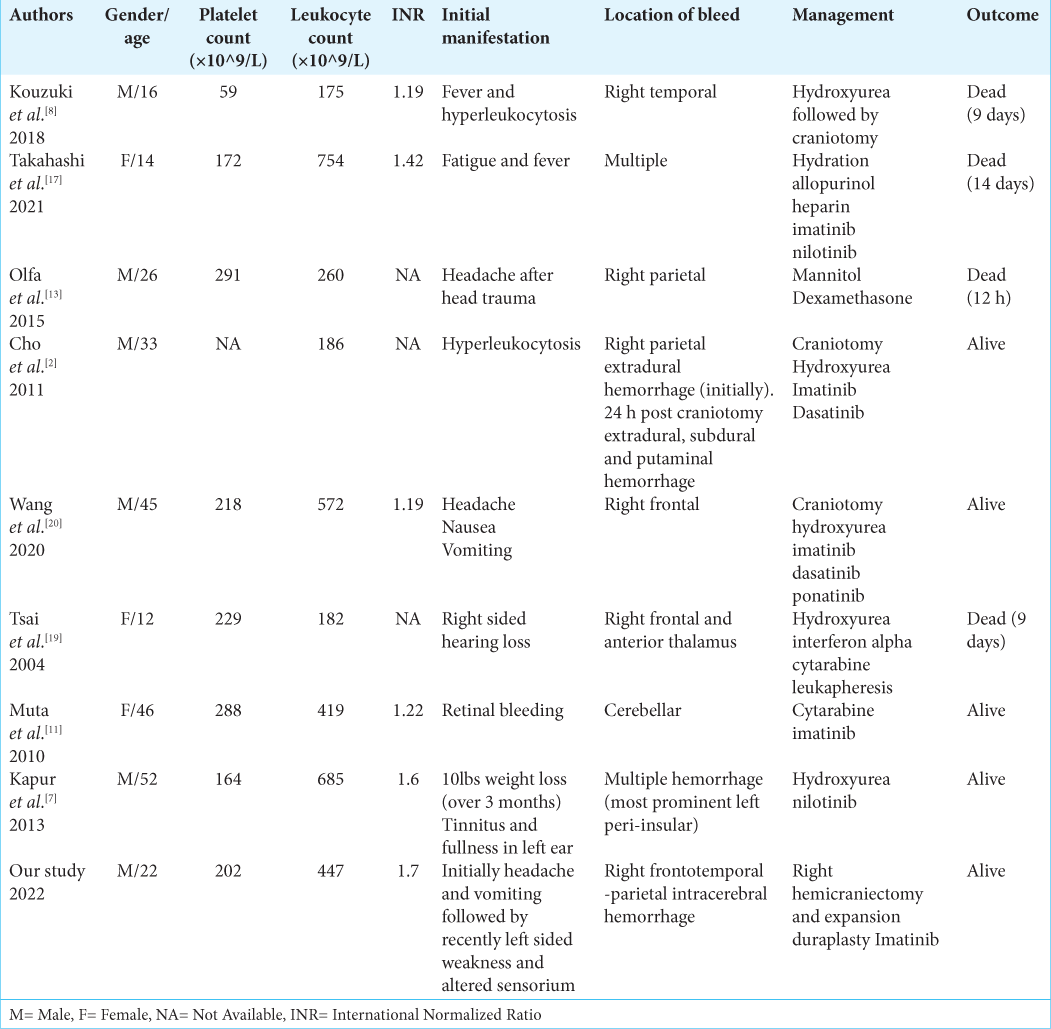
Based on the previous cases, in which craniotomies were performed, it was found that there is a high possibility of rebleeding in CML with ICH. However, some authors performed craniotomies for intracranial decompression to allow space for edema and to help confirm the diagnosis. Wang et al.[
Similarly, Cho et al.[
Cases where patients were not or could not be operated on include the following. Olfa et al.[
Following surgeries, patients have been treated with tyrosine kinase inhibitors which have revolutionized the treatment of CML and significantly improved overall survival by even decades.[
Wang et al.[
CONCLUSION
We report a rare presentation of CML-CP with an ICH causing the first symptomatic presentation. Intracranial involvement and ICH may be contributed to by leukostasis from hyperleukocytosis. When CML patients present with neurological symptoms, an urgent CT brain should be obtained. Rebleeding in CML patients is common, and they should be conservatively managed unless there is a need to alleviate intracranial pressure, in which case a craniotomy should be utilized. Patients should be monitored closely in neurosurgical high dependence and promptly be commenced oncological treatment with an appropriate TKI.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bonifacio M, Stagno F, Scaffidi L, Krampera M, Di Raimondo F. Management of chronic myeloid leukemia in advanced phase. Front Oncol. 2019. 9: 1132
2. Cho SF, Liu TC, Lu CY, Chang CS. Epidural leukemic involvement and intracranial hemorrhage as initial manifestations in a newly diagnosed chronic myeloid leukemia patient. Ann Hematol. 2011. 90: 607-9
3. Dayyani F, Mougalian SS, Naqvi K, Shan J, Ravandi F, Cortes J. Prediction model for mortality after intracranial hemorrhage in patients with leukemia. Am J Hematol. 2011. 86: 546-9
4. Gokce M, Unal S, Bayrakçı B, Tuncer M. Chronic myeloid leukemia presenting with visual and auditory impairment in an adolescent: An insight to management strategies. Indian J Hematol Blood Transfus. 2010. 26: 96-8
5. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. 2020. 95: 691-709
6. Kalmanti L, Saussele S, Lauseker M, Proetel U, Müller MC, Hanfstein B. Younger patients with chronic myeloid leukemia do well in spite of poor prognostic indicators: Results from the randomized CML study IV. Ann Hematol. 2014. 93: 71-80
7. Kapur S, Wax M, Miles L, Hussain A. Permanent sensorineural deafness in a patient with chronic myelogenous leukemia secondary to intracranial hemorrhage. Case Rep Hematol. 2013. 2013: 894141
8. Kouzuki K, Umeda K, Saida S, Kato I, Hiramatsu H, Funaki T. Sudden intracranial hemorrhage in a patient with atypical chronic myeloid leukemia in chronic phase. J Pediatr Hematol Oncol. 2018. 40: e553-6
9. Kurosawa H, Tanizawa A, Tono C, Watanabe A, Shima H, Ito M. Leukostasis in children and adolescents with chronic myeloid leukemia: Japanese pediatric leukemia/ lymphoma study group. Pediatr Blood Cancer. 2016. 63: 406-11
10. Lieu AS, Hwang SL, Howng SL, Chai CY. Brain tumors with hemorrhage. J Formos Med Assoc. 1999. 98: 365-7
11. Muta T, Sawada Y, Moriyama Y, Seike Y, Tokuyama T, Ueda Y. Chronic myeloid leukemia complicated with cerebellar hemorrhage and acute hydrocephalus successfully treated with imatinib and intensive supportive care. Rinsho Ketsueki. 2010. 51: 1769-74
12. Naunheim MR, Nahed BV, Walcott BP, Kahle KT, Soupir CP, Cahill DP. Diagnosis of acute lymphoblastic leukemia from intracerebral hemorrhage and blast crisis. A case report and review of the literature. Clin Neurol Neurosurg. 2010. 112: 575-7
13. Olfa CW, Imen R, Leila K, Hichem K, Adnane A, Mourad C. Diagnosis of chronic myeloid leukemia from acute intracerebral hemorrhage: A case report. J Acute Dis. 2015. 4: 252-4
14. Seiter K. Update of recent studies in chronic myeloid leukemia. J Hematol Oncol. 2009. 2: A2
15. Sharma SR, Dey B. Blast crisis of chronic myeloid leukemia initially presenting as severe acute intracerebral hemorrhage. J Family Med Prim Care. 2020. 9: 1266-9
16. Shiber JR, Fines RE. Cerebral hemorrhage due to hyperleukocytosis. J Emerg Med. 2011. 40: 674-77
17. Takahashi N, Sano H, Mochizuki K, Kobayashi S, Ohara Y, Kikuta A. Intracranial hemorrhage in a pediatric patient with chronic myeloid leukemia in chronic phase: A case report. Case Rep Oncol. 2021. 14: 525-30
18. Terasawa T, Dahabreh I, Trikalinos TA. BCR-ABL mutation testing to predict response to tyrosine kinase inhibitors in patients with chronic myeloid leukemia. PLoS Curr. 2010. 2: RRN1204
19. Tsai CC, Huang CB, Sheen JM, Wei HH, Hsiao CC. Sudden hearing loss as the initial manifestation of chronic myeloid leukemia in a child. Chang Gung Med J. 2004. 27: 629-33
20. Wang H, Cao F, Li J, Sun K, Jin J, Wang M. Intracerebral hemorrhage as the initial presentation of chronic myeloid leukemia: A case report and review of the literature. Front Neurol. 2020. 11: 571576