- Department of Neurosurgery, Faculty of Medicine, The University of Tokyo, and
- Pathology, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan.
Correspondence Address:
Satoru Miyawaki, Department of Neurosurgery, Faculty of Medicine, The University of Tokyo, Tokyo, Japan.
DOI:10.25259/SNI_71_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takahiro Tsuchiya1, Satoru Miyawaki1, Yuki Shinya1, Yu Teranishi1, Arisa Tomioka1, Sho Yamazawa2, Masahito Shin1, Nobuhito Saito1. Intracranial ancient schwannoma originating from vestibular nerve: A case report and review of the literature. 15-Apr-2022;13:143
How to cite this URL: Takahiro Tsuchiya1, Satoru Miyawaki1, Yuki Shinya1, Yu Teranishi1, Arisa Tomioka1, Sho Yamazawa2, Masahito Shin1, Nobuhito Saito1. Intracranial ancient schwannoma originating from vestibular nerve: A case report and review of the literature. 15-Apr-2022;13:143. Available from: https://surgicalneurologyint.com/surgicalint-articles/11540/
Abstract
Background: Ancient schwannoma (AS) is a subtype of schwannoma with degenerative features, which often progresses slowly over a long period of time. Intracranial AS is a rare benign tumor and there are no detailed reports of AS originating from the vestibular nerve.
Case Description: Herein, we present the case of a patient with the right vestibular schwannoma with multiple meningiomas and review three previous cases of intracranial AS. Near-total resection was performed for vestibular schwannoma and the pathological findings were AS (World Health Organization Grade I). Five months postoperatively, gamma knife radiosurgery was performed for a recurrent lesion of the right vestibular schwannoma in the internal auditory meatus. Although AS is known to be a benign pathology, there are cases of rapid growth and early recurrence, as the one presented here. The high Ki-67 index (up to 5%) and the presence of cysts may be related to the rapid progression of intracranial AS.
Conclusion: Therefore, careful follow-up is necessary even if adequate removal is achieved. In addition to pathological studies, the genetic background of intracranial AS warrants future investigations. Further accumulation of cases is necessary to clarify the clinical features of intracranial AS.
Keywords: Cyst, Intracranial ancient schwannoma, Pathology, Vestibular schwannoma
INTRODUCTION
Ancient schwannoma (AS) is a pathological subtype of schwannoma, first reported by Ackerman and Taylor.[
CASE REPORT
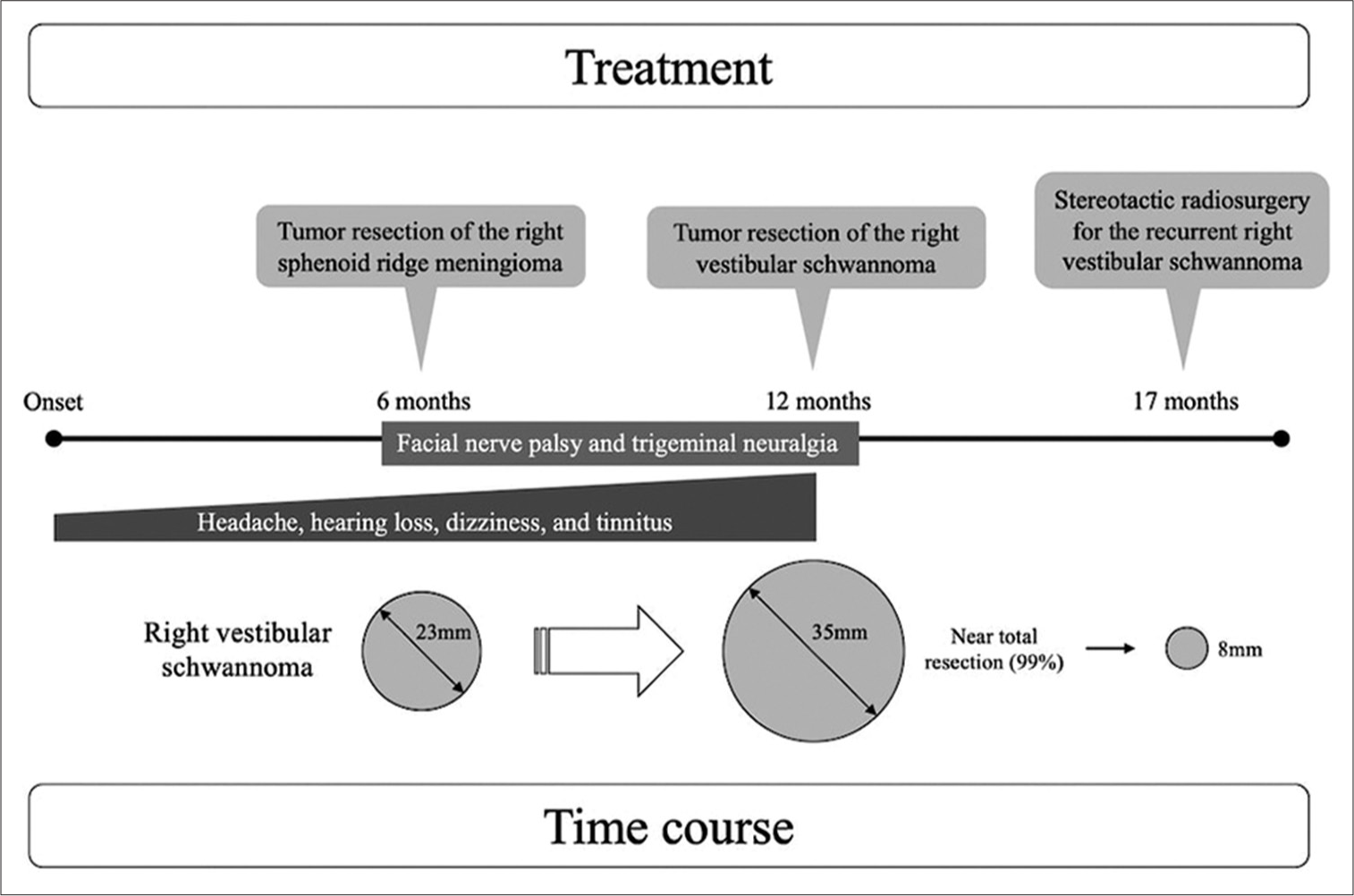
A 53-year-old woman was referred to our hospital with a history of gradually worsening symptoms of headache, hearing loss, dizziness, and tinnitus for half a year. MRI revealed a right cerebellopontine angle (CPA) tumor suspected to be a vestibular schwannoma, a right sphenoid ridge extra-axial tumor suspected as meningioma, and a left temporal extra-axial tumor also suspected to be a meningioma [
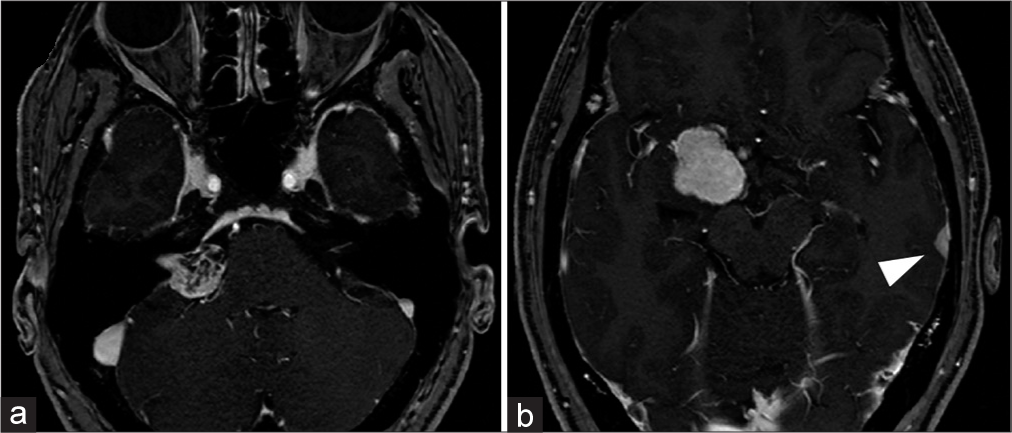
Figure 1:
Preoperative imaging of the patient. MRI revealed a right CPA tumor suspected to be a vestibular schwannoma (a), a right sphenoid ridge extra-axial tumor suspected to be meningioma (b), and a left temporal extra-axial tumor also suspected to be a meningioma (b, arrow head). MRI: magnetic resonance imaging, CPA: cerebellopontine angle.
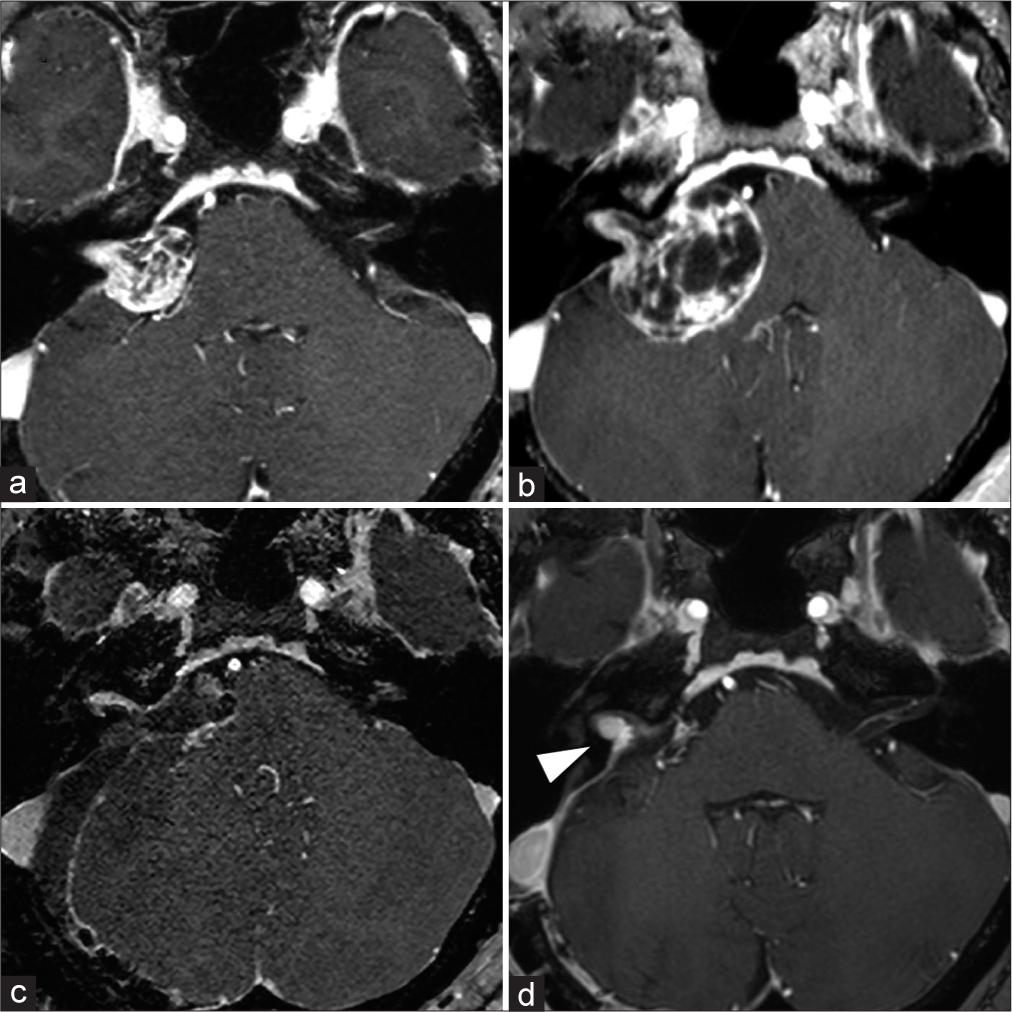
Figure 2:
Imaging follow-up of the right CPA tumor. The right CPA tumor grew rapidly, with its maximum diameter expanding from 23 mm (a) to 35 mm (b) in 6 months, and the brainstem compression worsened. MRI also showed a marked increase in the size of the cyst. MRI: magnetic resonance imaging, CPA: cerebellopontine angle. To avoid persistent facial nerve palsy, we left a tiny tumor on the facial nerve while performing near total resection (resection rate: 99%) (c). Five months postoperatively, the right residual vestibular schwannoma in the internal auditory meatus had grown, so stereotactic radio surgery was performed for the recurrent lesion (d, arrow).
We resected the right CPA tumor through a lateral suboccipital retrosigmoid approach. The tumor extended into the internal auditory meatus, the cochlear nerve was running on the caudal side of the tumor, and the facial nerve was located on its ventral side, as confirmed by electrophysiological monitoring. Intraoperative findings suggested that the tumor was a vestibular schwannoma. To avoid persistent facial nerve palsy, we left a tiny tumor on the facial nerve while performing near-total resection (resection rate: 99%; [
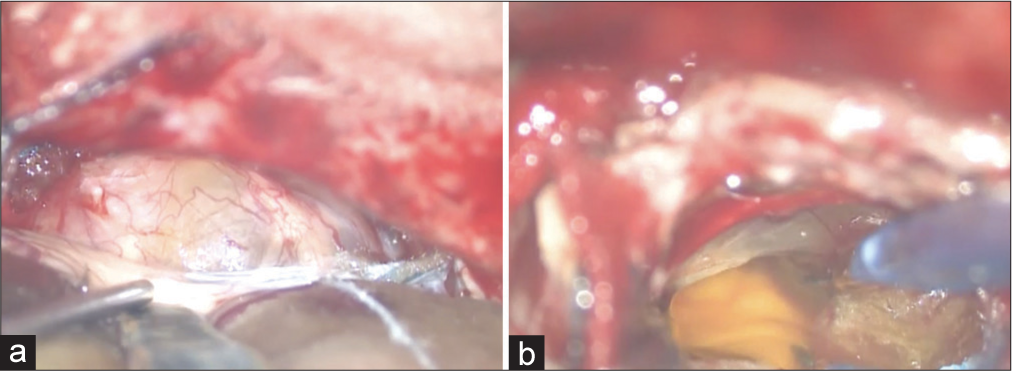
Intraoperative images of tumor resection are shown in [
Figure 3:
Intraoperative images of tumor resection. The tumor extended into the internal auditory meatus, the cochlear nerve was running on the caudal side of the tumor, and the facial nerve was located on its ventral side, which was confirmed by electrophysiological monitoring (a). The tumor was soft and had a cystic component (b).
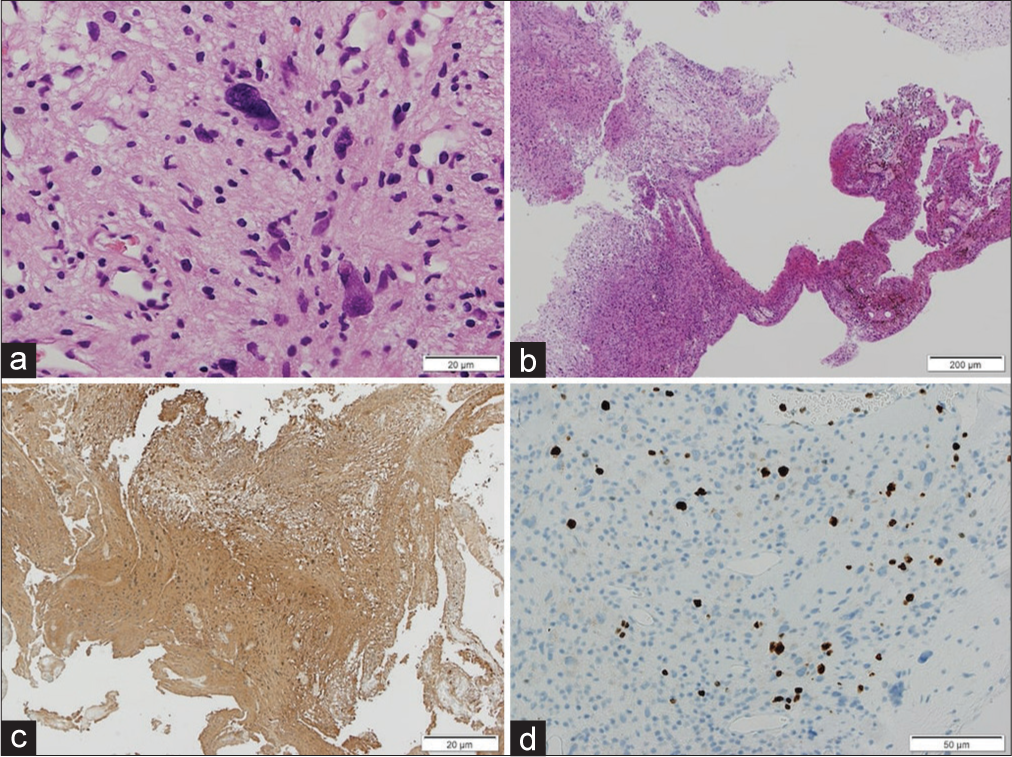
Figure 4:
Histopathological findings of the tumor. Atypical Schwann cells with nuclear pleomorphism were observed, but mitotic figures were rarely seen (a). Degenerative changes such as hyalinized blood vessels, hemorrhage with hemosiderin deposition, lymphocytic infiltration, and cyst formation (b) were observed. Immunostaining showed diffuse S100 positivity (c) and the Ki-67 proliferation index was up to 5% (d). The diagnosis was ancient schwannoma (WHO Grade I). WHO: World Health Organization.
A detailed timeline on the treatment and the time course is presented in [
DISCUSSION
We report a case of a rapidly progressing ancient vestibular schwannoma with multiple meningiomas.[
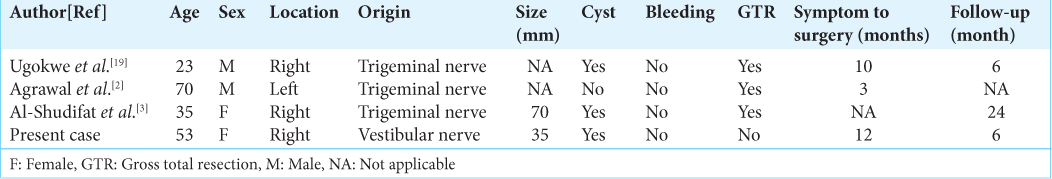
We reviewed four cases of intracranial AS, including our case [
Extracranial AS is known to be characterized by a long duration from symptom onset to surgery, while intracranial AS may cause nerve compression at an early stage as the tumor progresses. It has been reported that the average time from symptoms to surgery in patients with extracranial AS is 8.3 years;[
Cystic vestibular schwannoma is known to have larger tumor size, more rapid growth, and shorter duration of symptoms compared to solid vestibular schwannoma.[
It is well known that the central cystic remodeling can be observed in large schwannomas. While differentiating AS from other subtypes with cystic remodeling, AS is characterized by nuclear atypia or degenerative changes on microscopy. Cysts are a degenerative finding but only one feature of the AS. On the other hand, MRI features are not well defined for AS. Hence, currently, diagnosing AS from MRI findings is difficult in the preoperative phase when pathology specimens have not been obtained, but the findings of cysts may increase the possibility of an AS diagnosis.
The pathological diagnosis is crucial; however, pathologists must be careful in their diagnosis because the nuclear atypia and degenerative features may lead AS to be misdiagnosed as malignant.[
Since the present patient had unilateral VS and multiple meningiomas, she was diagnosed with neurofibromatosis Type 2 (NF2) according to the latest NF2 diagnostic criteria (Manchester criteria).[
CONCLUSION
Intracranial AS is rare, so further accumulation and review of cases are necessary to clarify its clinical features. Although AS is known to be a benign pathology, there are cases of rapid growth and early recurrence, as the one presented here. The high Ki-67 index and the presence of cysts may be related to the rapid progression of intracranial AS. Therefore, careful follow-up is necessary even if adequate removal is achieved. In addition to pathological studies, the genetic background of intracranial AS warrants future investigations.
Statements and declarations
This manuscript is original and has not been published or presented elsewhere in part or in whole.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ackerman LV, Taylor FH. Neurogenous tumors within the thorax; a clinicopathological evaluation of forty-eight cases. Cancer. 1951. 4: 669-91
2. Agrawal A, Bhake A, Meshram N. Ancient schwannoma of the trigeminal nerve mimicking high grade lesion. Iran J Pathol. 2010. 5: 97-9
3. Al-Shudifat A, Mafrachi B, Al-Ani A, Qaisi AK, Bustami N. Ancient schwannoma affecting the intracranial portion of the trigeminal nerve: A case report. Surg Neurol Int. 2020. 11: 426
4. Argenyi ZB, Balogh K, Abraham AA. Degenerative (“ancient”) changes in benign cutaneous schwannoma. A light microscopic, histochemical and immunohistochemical study. J Cutan Pathol. 1993. 20: 148-53
5. Çalişkan S, Gümrükçü G, Kaya C. Retroperitoneal ancient schwannoma: A case report. Rev Urol. 2015. 17: 190-3
6. Evans DG, Huson SM, Donnai D, Neary W, Blair V, Newton V. A clinical study of Type 2 neurofibromatosis. Q J Med. 1992. 84: 603-18
7. Gomez-Brouchet A, Delisle MB, Cognard C, Bonafe A, Charlet JP, Deguine O. Vestibular schwannomas: Correlations between magnetic resonance imaging and histopathologic appearance. Otol Neurotol. 2001. 22: 79-86
8. Hirose T, Ishizawa K, Sakaki M, Fujii Y. Retroperitoneal schwannoma is characterized by a high incidence of cellular type and GFAP-immunoreactivity. Pathol Int. 2012. 62: 456-62
9. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985. 93: 146-7
10. Isobe K, Shimizu T, Akahane T, Kato H. Imaging of ancient schwannoma. AJR Am J Roentgenol. 2004. 183: 331-6
11. Jian BJ, Sughrue ME, Kaur R, Rutkowski MJ, Kane AJ, Kaur G. Implications of cystic features in vestibular schwannomas of patients undergoing microsurgical resection. Neurosurgery. 2011. 68: 874-80
12. Malizos K, Ioannou M, Kontogeorgakos V. Ancient schwannoma involving the median nerve: A case report and review of the literature. Strateg Trauma Limb Reconstr. 2013. 8: 63-6
13. Moon KS, Jung S, Seo SK, Jung TY, Kim IY, Ryu HH. Cystic vestibular schwannomas: A possible role of matrix metalloproteinase-2 in cyst development and unfavorable surgical outcome. J Neurosurg. 2007. 106: 866-71
14. Park CK, Kim DC, Park SH, Kim JE, Paek SH, Kim DG. Microhemorrhage, a possible mechanism for cyst formation in vestibular schwannomas. J Neurosurg. 2006. 105: 576-80
15. Ratnagiri R, Mallikarjun S. Retroperitoneal ancient schwannoma: Two cases and review of literature. J Cancer Res Ther. 2014. 10: 368-70
16. Shanmugasundaram G, Thangavel P, Venkataraman B, Barathi G. Incidental ancient schwannoma of the posterior mediastinum in a young male: A rare scenario. BMJ Case Rep. 2019. 12: e227497
17. Sinha S, Sharma BS. Cystic acoustic neuromas: Surgical outcome in a series of 58 patients. J Clin Neurosci. 2008. 15: 511-5
18. Teranishi Y, Miyawaki S, Hongo H, Dofuku S, Okano A, Takayanagi S. Targeted deep sequencing of DNA from multiple tissue types improves the diagnostic rate and reveals a highly diverse phenotype of mosaic neurofibromatosis Type 2. J Med Genet. 2021. 58: 701-11
19. Ugokwe K, Nathoo N, Prayson R, Barnett GH. Trigeminal nerve schwannoma with ancient change. J Neurosurg. 2005. 102: 1163-5
20. Zhou J, Zhang D, Li W, Zhou L, Xu H, Zheng S. Primary adrenal schwannoma: A series of 31 cases emphasizing their clinicopathologic features and favorable prognosis. Endocrine. 2019. 65: 662-74