- Department of Neurosurgery, Princess Alexandra Hospital, Woolloongabba, QLD, Australia.
Correspondence Address:
John Carbone, Department of Neurosurgery, Princess Alexandra Hospital, Woolloongabba, 4102 Queensland, Australia.
DOI:10.25259/SNI_946_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: John Carbone, Ananthababu Pattavilakom Sadasivan. Intracranial arachnoid cysts: Review of natural history and proposed treatment algorithm. 20-Dec-2021;12:621
How to cite this URL: John Carbone, Ananthababu Pattavilakom Sadasivan. Intracranial arachnoid cysts: Review of natural history and proposed treatment algorithm. 20-Dec-2021;12:621. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11297
Abstract
Background: With a prevalence of 1.4%, intracranial arachnoid cysts are a frequent incidental finding on MRI and CT. Whilst most cysts are benign in the long-term, clinical practice, and imaging frequency does not necessarily reflect this.
Methods: A literature review was conducted searching the Medline database with MESH terms. This literature was condensed into an article, edited by a consultant neurosurgeon. This was further condensed, presented to the neurosurgery department at Princess Alexandra Hospital for final feedback and editing.
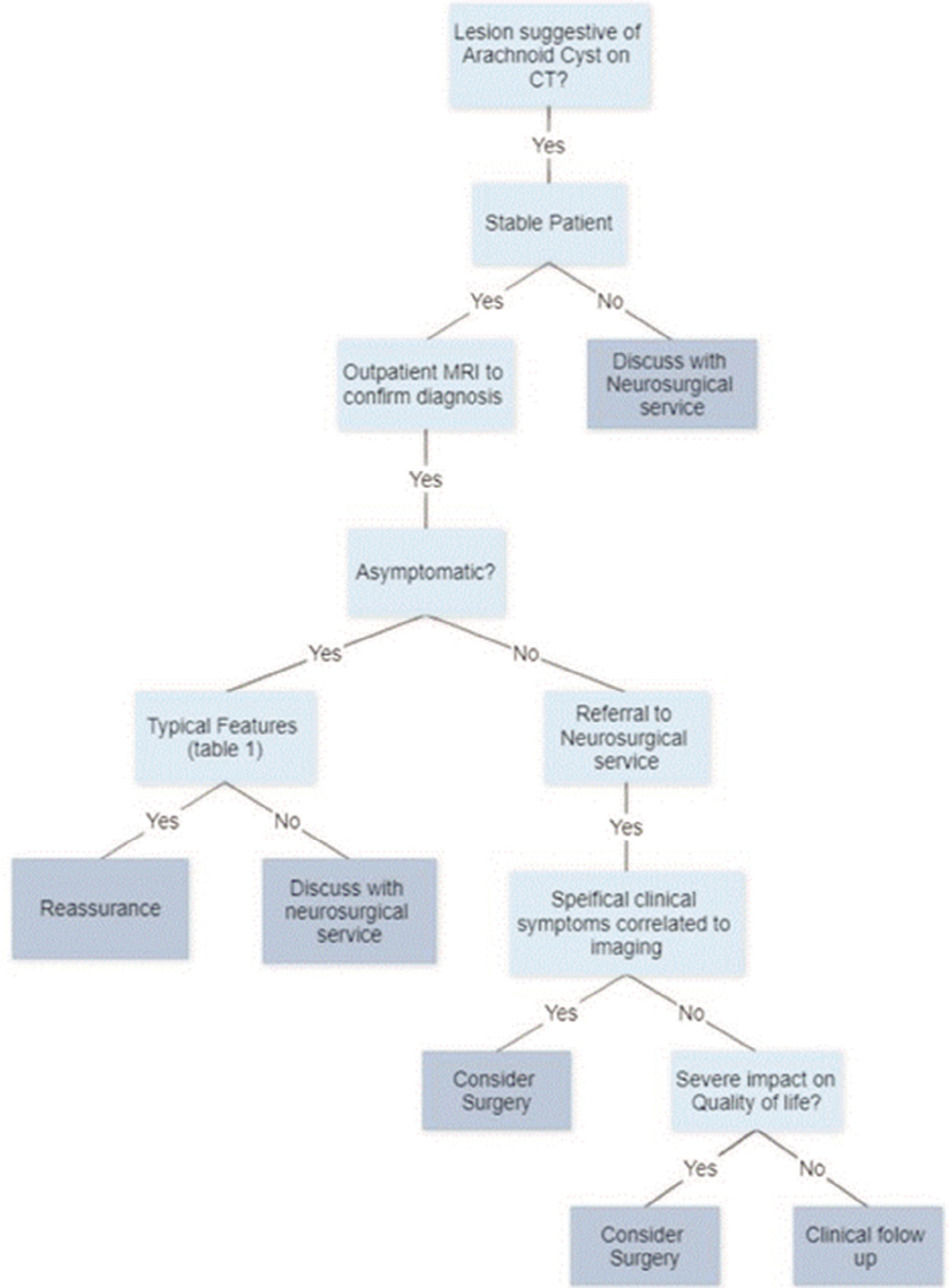
Results: This review advises that asymptomatic patients with typical cysts have a low risk of cyst growth and development of new symptomatology, thus do not require surveillance or intervention. The minority of symptomatic patients or those with cysts in sensitive areas may require referral to a neurosurgeon for clinical follow-up or intervention.
Conclusion: Greater than 94% of patients are asymptomatic, practitioners can be confident in reassuring patients of the benign nature of a potentially worrying finding. Recognizing the small number of symptomatic patients and those with cysts in areas sensitive to causing hydrocephalus is where GP decision making in conjunction with specialty input is of highest yield.
Keywords: Arachnoid cyst, Communication, General practice, Neurosurgery, Treatment algorithm
INTRODUCTION
Intracranial arachnoid cysts are collections of cerebrospinal fluid (CSF) encased in a layer of collagen and arachnoidal cells.[
Aim
This article aims to improve risk stratification skills of General Practitioners (GPs) in their daily practice, reduce unnecessary imaging, increase confidence in reassuring the majority of patients, and provide appropriate access to tertiary care for patients who may require intervention or follow-up. Recognizing the small number of symptomatic patients and those with cysts in areas sensitive to causing hydrocephalus is where GP decision making in conjunction with specialty input is of highest yield.
ETIOLOGY/PATHOGENESIS
Arachnoid cysts are thought to form through splitting of the arachnoid membrane, allowing for abnormal collection of fluid. They may occur anywhere along the neuro-axis from head to spine. Histopathologically, the cysts demonstrate splitting of the arachnoid membrane at the margin of the cyst, thick collagen layers, absent trabecular processes, and hyperplastic arachnoid cells in the cyst wall.[
EPIDEMIOLOGY
Estimates of cyst prevalence in adults have historically been 1%.[
IMAGING
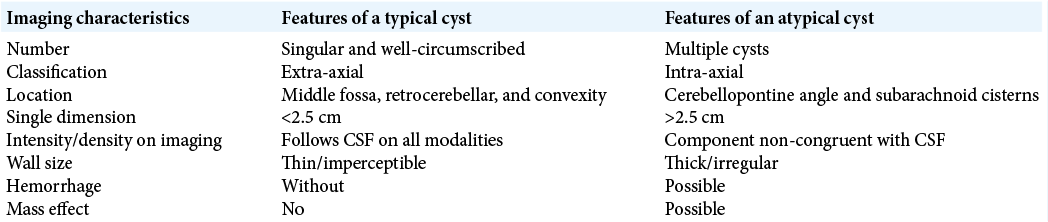
CT and MR studies demonstrate that intracranial arachnoid cysts are well-circumscribed, extra-axial, and simple cystic lesions. They are isodense to CSF on CT, and isointense to CSF on all MRI sequences.[
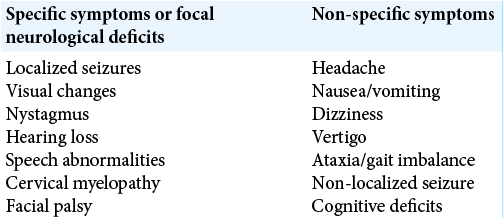
CLINICAL PRESENTATION
Greater than 94%[
ASYMPTOMATIC PATIENTS
Given the benign nature of the majority of adult arachnoid cysts, asymptomatic patients with typical cysts have a low risk of cyst growth and development of new symptomatology.[
SYMPTOMATIC PATIENTS
Diagnosis
Correlating an arachnoid cyst with clinical presentation is a subjective assessment, with no objective test available to the surgeon. Symptom severity, cyst size, and correlating location with the presenting complaint are the best tools available to the present practitioners.
Surgical treatment
The aim of intervention is to achieve decompression of the cyst and establish communication between the normal and pathological CSF spaces. In general, the present evidence suggests that the treatment of symptomatic arachnoid cysts with surgical intervention does appear effective. A recent meta-analysis by Hayes et al. conducted by a literature search of existing studies concluded the treatment effect was 0.667 (P < 0.01) for all surgical intervention in improving patient outcomes.[
Is there a best treatment?
Two primary interventions are available for treating arachnoid cysts surgically, open craniotomy or endoscopic fenestration. The technique chosen is generally determined by surgeon experience and cyst location. There is no consensus for which method is best. Meta-analysis of retrospectively analyzed surgical methods shows similar rates of positive treatment effect between all practices.[
Craniotomy theoretically favors cysts in which the surrounding arachnoid is compressed,[
CONCLUSION
This review concludes that asymptomatic patients with typical cysts have a low risk of cyst growth and development of new symptomatology, thus do not require surveillance or intervention. Greater than 94%[
KEY POINTS
Majority of arachnoid cysts are asymptomatic and are unlikely to become symptomatic. Surveillance imaging is not required as they are unlikely to change size. Cysts in sensitive areas or symptomatic patients should be discussed with a neurosurgical service. Atypical cysts should be discussed with a neurosurgical service. Surgery appears to have a role in patients whose symptoms can be clinically ascribed to a symptomatic cyst.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Holou WN, Terman S, Kilburg C, Garton HJ, Muraszko KM, Maher CO. Prevalence and natural history of arachnoid cysts in adults. J Neurosurg. 2013. 118: 222-31
2. Basaldella L, Orvieto E, Dei Tos AP, Della Barbera M, Valente M, Longatti P. Causes of arachnoid cyst development and expansion. Neurosurg Focus. 2007. 22: E4
3. Berle M, Wester KG, Ulvik RJ, Kroksveen AC, Haaland OA, Amiry-Moghaddam M. Arachnoid cysts do not contain cerebrospinal fluid: A comparative chemical analysis of arachnoid cyst fluid and cerebrospinal fluid in adults. Cerebrospinal Fluid Res. 2010. 7: 8
4. Chen Y, Fang HJ, Li ZF, Yu SY, Li CZ, Wu ZB. Treatment of middle cranial fossa arachnoid cysts: A systematic review and meta-analysis. World Neurosurg. 2016. 92: 480-90.e2
5. Cinalli G, Spennato P, Columbano L, Ruggiero C, Aliberti F, Trischitta V. Neuroendoscopic treatment of arachnoid cysts of the quadrigeminal cistern: A series of 14 cases. J Neurosurg Pediatr. 2010. 6: 489-97
6. Gangemi M, Colella G, Magro F, Maiuri F. Suprasellar arachnoid cysts: Endoscopy versus microsurgical cyst excision and shunting. Br J Neurosurg. 2007. 21: 276-80
7. Gangemi M, Seneca V, Colella G, Cioffi V, Imperato A, Maiuri F. Endoscopy versus microsurgical cyst excision and shunting for treating intracranial arachnoid cysts. J Neurosurg Pediatr. 2011. 8: 158-64
8. Gui S, Bai J, Wang X, Zong X, Li C, Cao L. Assessment of endoscopic treatment for quadrigeminal cistern arachnoid cysts: A 7-year experience with 28 cases. Childs Nerv Syst. 2016. 32: 647-54
9. Hall A, White MA, Myles L. Spontaneous subdural haemorrhage from an arachnoid cyst: A case report and literature review. Br J Neurosurg. 2017. 31: 607-10
10. Hall S, Smedley A, Sparrow O, Mathad N, Waters R, Chakraborty A. Natural history of intracranial arachnoid cysts. World Neurosurg. 2019. 126: e1315-20
11. Hayes MJ, TerMaath SC, Crook TR, Killeffer JA. A review on the effectiveness of surgical intervention for symptomatic intracranial arachnoid cysts in adults. World Neurosurg. 2019. 123: e259-72
12. Heier LA, Zimmerman RD, Amster JL, Gandy SE, Deck MD. Magnetic resonance imaging of arachnoid cysts. Clin Imaging. 1989. 13: 281-91
13. Helland CA, Wester K. Intracystic pressure in patients with temporal arachnoid cysts: A prospective study of preoperative complaints and postoperative outcome. J Neurol Neurosurg Psychiatry. 2007. 78: 620-3
14. Moss T, Helland CA, Mørkve SH, Wester K. Surgical decompression of arachnoid cysts leads to improved quality of life: A prospective study-long-term follow-up. Acta Neurochir (Wien). 2019. 161: 2253-63
15. Osborn AG, Preece MT. Intracranial cysts: Radiologicpathologic correlation and imaging approach. Radiology. 2006. 239: 650-64
16. Rabiei K, Hellström P, Högfeldt-Johansson M, Tisell M. Does subjective improvement in adults with intracranial arachnoid cysts justify surgical treatment?. J Neurosurg. 2018. 128: 250-7
17. Rengachary SS, Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol. 1981. 40: 61-83
18. Robinson RG. Congenital cysts of the brain: Arachnoid malformations. Prog Neurol Surg. 1971. 4: 133-74
19. Shim KW, Lee YH, Park EK, Park YS, Choi JU, Kim DS. Treatment option for arachnoid cysts. Childs Nerv Syst. 2009. 25: 1459-66