- Department of Neurology, University of Alabama at Birmingham, Birmingham, Alabama, USA
- Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama, USA
- Dartmouth–Hitchcock Medical Center, Lebanon, New Hampshire, USA
- Department of Pathology, University of Utah, Salt Lake City, Utah, USA
- Department of Pathology, University of Alabama at Birmingham, Birmingham, Alabama, USA
Correspondence Address:
Ross L. Dawkins
Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama, USA
DOI:10.4103/2152-7806.170025
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Arora K, Dawkins RL, Bauer DF, Palmer CA, Hackney JR, Markert JM. Intracranial blastomycotic abscess mimicking malignant brain neoplasm: Successful treatment with voriconazole and surgery. Surg Neurol Int 20-Nov-2015;6:174
How to cite this URL: Arora K, Dawkins RL, Bauer DF, Palmer CA, Hackney JR, Markert JM. Intracranial blastomycotic abscess mimicking malignant brain neoplasm: Successful treatment with voriconazole and surgery. Surg Neurol Int 20-Nov-2015;6:174. Available from: http://surgicalneurologyint.com/surgicalint_articles/intracranial-blastomycotic-abscess-mimicking-malignant-brain/
Abstract
Background:Cerebral blastomycosis is a rarely reported disease, and in the absence of associated, underlying systemic infection, poses a great diagnostic difficulty. Magnetic resonance imaging can sometimes provide equivocal information when trying to pinpoint a diagnosis. Classically, cerebral blastomycosis has been treated with amphotericin B. Voriconazole is a newer triazole antifungal with potential as a follow-up treatment of blastomycosis of the central nervous system after initial therapy with amphotericin B.
Case Description:We describe one such case of a cerebral blastomycotic abscess, presenting in the absence of any systemic disease, which was initially thought to be a neoplasm. It was successfully treated by surgical resection followed by sequential amphotericin B and voriconazole. The patient did well with voriconazole therapy and was followed for voriconazole tolerance with liver function tests, which continued to be stable at 8 months past the initiation of therapy. At 12 months postoperatively, the patient was doing well and showed gradual improvement in a visual field cut, with no sign of recurrent infection.
Conclusions:Isolated cerebral blastomycosis can present a diagnostic challenge. In the absence of systemic infection, surgical resection followed by antifungal therapy is a logical treatment plan.
Keywords: Blastomycotic abscess, cerebral blastomycosis, voriconazole
INTRODUCTION
Blastomycosis is an uncommon but often serious mycosis, endemic in the Southeastern and the Central United States. Approximately, 800 hospitalizations occur annually in the United States for blastomycosis, of which 6% result in death.[
Once diagnosed, intracranial blastomycosis has classically been treated with amphotericin B deoxycholate or lipid formulations of amphotericin B.[
This report discusses a case of a 37 year old, otherwise healthy, nondiabetic, and immunocompetent, white man presenting with an isolated left temporo-occipital mass lesion, identified as Blastomyces dermatitidis, and successfully treated with surgical resection, followed by the sequential use of amphotericin B and voriconazole.
HISTORY
A 37-year-old, ambidextrous taxidermist presented to ophthalmology clinic at the University of Alabama at Birmingham Hospital with a several year history of visual floaters of the right eye and intermittent decreased hearing on the left. He complained of worsening symptoms over the previous 8 months with intermittent sensations of pressure behind the right eye, difficulty with balance and ambulation, difficulty reading, and mild headaches. He also described poorly defined problems with cognition. He did not report the impairment of speech, language, or comprehension. His past medical and surgical histories were unremarkable. He denied the use of tobacco or intravenous drugs and indicated infrequent alcohol use.
Examination
On physical examination, significant findings included a small right inferior quadrantanopia to confrontational examination. Automated visual fields documented the presence of a congruous inferior quadrantanopia. His neurologic examination was otherwise normal. No abnormal skin findings were present.
Work-up
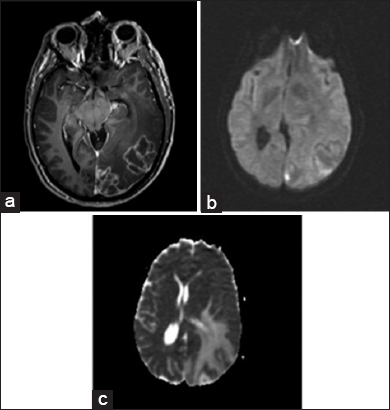
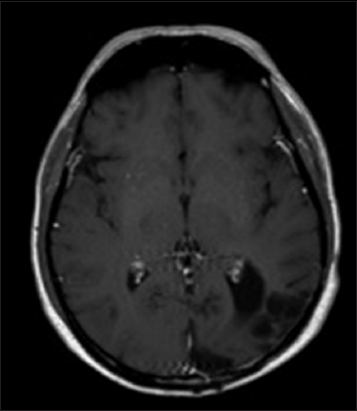
An magnetic resonance imaging (MRI) of the brain was obtained and revealed two closely associated large, heterogeneous, left temporo-occipital lesions with mass effect. The lesions were heterogeneously enhancing on T1-weighted images, and T2-weighted images showed changes consistent with edema extending through the temporal and occipital lobes. Diffusion-weighted imaging demonstrated equivocal increased signal intensity within the lesions. A low apparent diffusion coefficient (ADC) was not seen on the ADC map, as is typical with a cerebral abscesses. While closely associated with the convexity dura and the tentorium, the masses appeared to be intra-axial, and were interpreted as most consistent with malignant glioma or metastatic lesion [
Figure 1
Preoperative axial images. T1-weighted image after gadolinium administration (a), demonstrating multiloculated ring-enhancement. Diffusion- weighted image demonstrates a very small amount of increased signal (b), but a decreased apparent diffusion coefficient value is not obvious (c). Findings are consistent with malignant neoplasm, either primary or metastatic, but also with abscess
Operation and initial treatment
For diagnosis and therapy, the patient was taken to the operating room for a neuronavigation-guided resection of the mass via a temporo-occipital craniotomy with ultrasound assistance in delineating the borders of the lesion. The intra-operative specimen appeared to be a fungal abscess on frozen section evaluation. The abscess was resected [
Neuropathology
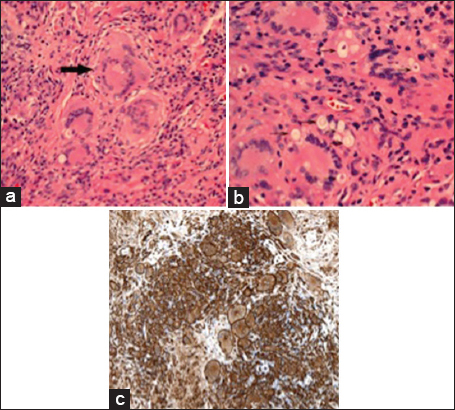
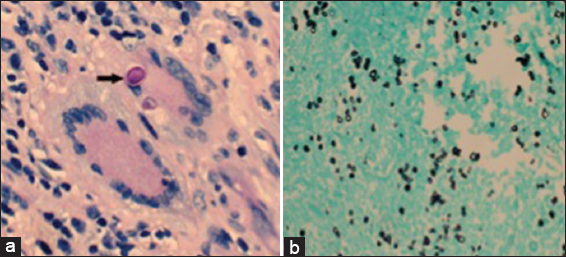
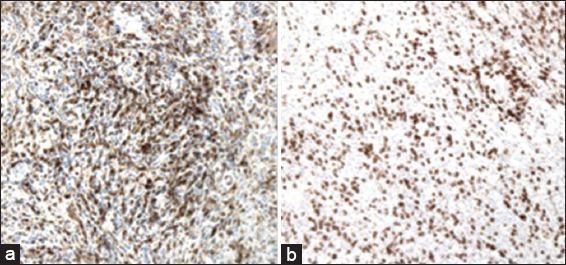
Histopathology revealed a granulomatous process with encapsulated yeast forms. Numerous multinucleated giant cells were present, some of which contained fungal organisms, which were small, refractile, and variably-sized. A CD163 stain demonstrated abundant macrophages, and also highlighted the larger, multinucleated histiocytic giant cells [
Follow-up
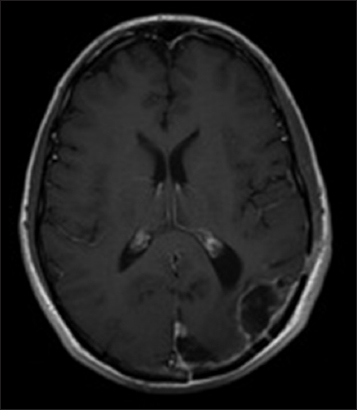
The patient did well with voriconazole therapy and was followed up for possible voriconazole toxicity with liver function tests, which continued to be stable at 8 months past the initiation of therapy. Due to the development of photosensitivity and some areas of cutaneous erythema, voriconazole was stopped after 7 months of therapy and switched to oral fluconazole 800 mg daily. This was continued for 3 months. At 12 months postoperatively, the patient was doing well and showed gradual improvement in his visual field deficit. His MRI at this time showed small lobulated areas of enhancement that were interpreted as postsurgical change and not a recurrence of the abscess [
DISCUSSION
Blastomycosis is a systemic fungal infection caused by a dimorphic fungus B. dermatitidis. A disease of worldwide distribution, it is found to be endemic in the Ohio and Mississippi river valleys of the United States, as well as Central Canada.
It is primarily a disease of the lung, though secondary dissemination to skin, bones genito-urinary tract, and rarely CNS has been documented.[
The guidelines published by the Mycosis Study Group under the auspices of the Infectious Disease Society of America in 2008 recommend the intravenous amphotericin B lipid formulations as first line treatment for CNS blastomycosis.[
While an isolated visual field deficit can be difficult to detect if it is subtle, this case report emphasizes the importance of full visual field examination as part of a neurological examination. While we do not believe that the abscess was related to some of the patient's initial complaints, such as the visual floaters and decreased hearing on the left, we are certain that the lesion does explain the visual field deficits found on examination. A congruous and right sided quadrantanopia fits with a lesion in the left occipital lobe, which was the location of our patient's abscess. Furthermore, our patient had improvement in visual fields after the resection of the abscess. MRI evaluation of blastomycosis can be difficult, and these lesions can appear consistent with intracranial malignancies or other infectious conditions. In addition, it is rare, but possible, to have an intracranial fungal abscess without systemic disease. Our patient had no risk factors for infection nor any evidence of any systemic malignancy and thus surgical treatment was the logical approach to both diagnosis and treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bakleh M, Aksamit AJ, Tleyjeh IM, Marshall WF. Successful treatment of cerebral blastomycosis with voriconazole. Clin Infect Dis. 2005. 40: e69-71
2. Bariola JR, Perry P, Pappas PG, Proia L, Shealey W, Wright PW. Blastomycosis of the central nervous system: A multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis. 2010. 50: 797-804
3. Bell RM, Starshak RJ, Sty JR, Harb JM. Solitary intracranial blastomycotic abscess. Wis Med J. 1983. 82: 23-5
4. Borgia SM, Fuller JD, Sarabia A, El-Helou P. Cerebral blastomycosis: A case series incorporating voriconazole in the treatment regimen. Med Mycol. 2006. 44: 659-64
5. Chapman SW, Dismukes WE, Proia LA, Bradsher RW, Pappas PG, Threlkeld MG. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008. 46: 1801-12
6. Chapman SW, Mandell GL, Douglas GR, Bennett JE.editors. Blastomyces dermatitidis. Infectious Diseases and Their Etiologic Agents. Principles and Practice of Infectious Disease. New York: Churchill Livingstone; 1990. p. 1999-2000
7. Chu JH, Feudtner C, Heydon K, Walsh TJ, Zaoutis TE. Hospitalizations for endemic mycoses: A population-based national study. Clin Infect Dis. 2006. 42: 822-5
8. Freifeld A, Proia L, Andes D, Baddour LM, Blair J, Spellberg B. Voriconazole use for endemic fungal infections. Antimicrob Agents Chemother. 2009. 53: 1648-51
9. Gonyea EF. The spectrum of primary blastomycotic meningitis: A review of central nervous system blastomycosis. Ann Neurol. 1978. 3: 26-39
10. Kravitz GR, Davies SF, Eckman MR, Sarosi GA. Chronic blastomycotic meningitis. Am J Med. 1981. 71: 501-5
11. Lutsar I, Roffey S, Troke P. Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin Infect Dis. 2003. 37: 728-32
12. Morgan D, Young RF, Chow AW, Mehringer CM, Itabashi H. Recurrent intracerebral blastomycotic granuloma: Diagnosis and treatment. Neurosurgery. 1979. 4: 319-24
13. Morse HG, Nichol WP, Cook DM, Blank NK, Ward TT. Central nervous system and genitourinary blastomycosis: Confusion with tuberculosis. West J Med. 1983. 139: 99-103
14. Panicker J, Walsh T, Kamani N. Recurrent central nervous system blastomycosis in an immunocompetent child treated successfully with sequential liposomal amphotericin B and voriconazole. Pediatr Infect Dis J. 2006. 25: 377-9
15. Roos KL, Bryan JP, Maggio WW, Jane JA, Scheld WM. Intracranial blastomycoma. Medicine Baltimore. 1987. 66: 224-35
16. Treseler CB, Sugar AM. Fungal meningitis. Infect Dis Clin North Am. 1990. 4: 789-808
17. Turner G, Scaravilli F, Graham D, Lantos PL.editors. Parasitic and fungal disease: Blastomycosis. Greenfield's Neuropathology. London: Arnold; 2002. p. 34-35
18. Ward BA, Parent AD, Raila F. Indications for the surgical management of central nervous system blastomycosis. Surg Neurol. 1995. 43: 379-88
19. Wylen EL, Nanda A. Blastomyces dermatitidis occurring as an isolated cerebellar mass. Neurosurg Rev. 1999. 22: 152-4