- Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia
- Department of Neurology, Massachusetts General Hospital, Boston, MA, USA
- Department of Neurology, Emory University School of Medicine, Atlanta, Georgia
Correspondence Address:
Raul G. Nogueira
Department of Neurology, Emory University School of Medicine, Atlanta, Georgia
DOI:10.4103/2152-7806.179577
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Baum GR, Turan N, Buonanno FS, Pradilla G, Nogueira RG. Intracranial dural arteriovenous fistula as a cause for symptomatic superficial siderosis: A report of two cases and review of the literature. Surg Neurol Int 01-Apr-2016;7:
How to cite this URL: Baum GR, Turan N, Buonanno FS, Pradilla G, Nogueira RG. Intracranial dural arteriovenous fistula as a cause for symptomatic superficial siderosis: A report of two cases and review of the literature. Surg Neurol Int 01-Apr-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/intracranial-dural-arteriovenous-fistula-as-a-cause-for-symptomatic-superficial-siderosis-a-report-of-two-cases-and-review-of-the-literature/
Abstract
Background:Superficial siderosis (SS) is the occult deposition of hemosiderin within the cerebral cortex due to repeat microhemorrhages within the central nervous system. The collection of hemosiderin within the pia and superficial cortical surface can lead to injury to the nervous tissue. The most common presentation is occult sensorineural hearing loss although many patients have been misdiagnosed with diseases such as multiple sclerosis and amyotrophic lateral sclerosis before being diagnosed with SS. Only one case report exists in the literature describing an intracranial dural arteriovenous fistula (dAVF) as the putative cause for SS.
Case Description:We describe two cases of SS caused by a dAVF. Both patients had a supratentorial, cortical lesion supplied by the middle meningeal artery with venous drainage into the superior sagittal sinus. In both patients, symptoms improved after endovascular embolization. The similar anatomic relationship of both dAVFs reported presents an interesting question about the pathogenesis of SS. Similar to the pathologic changes seen in the formation of intracranial arterial aneurysms; it would be possible that changes in the blood vessel lining and wall might predispose a patient to chronic, microhemorrhage resulting in SS.
Conclusions:We describe the second and third cases of a dAVF as the cause of SS, and the first cases of successful treatment of SS-associated dAVF with endovascular embolization. As noninvasive imaging techniques become more sensitive and easily obtained, one must consider their limitations in detecting occult intracranial vascular malformations such as dAVF as a possible etiology for SS.
Keywords: Dural arteriovenous fistula, embolization, superficial siderosis
INTRODUCTION
Superficial siderosis (SS) occurs as a result of hemosiderin deposition along leptomeninges, pial, subpial, and subependymal tissues due to recurrent microhemorrhages within the central nervous system (CNS).[
SS is seen in nearly half of the patients after single episode of high grade aneurysmal subarachnoid hemorrhage.[
Before the advent of magnetic resonance imaging (MRI), SS was diagnosed in postmortem studies. However, with the wide availability of MRI in current practice, the SS is more frequently being diagnosed. Pathognomic finding of hemosiderin deposition can be characterized as hypointensity on T2-weighted imaging (T2WI) MRI and gradient recalled echo T2-WI (GRE T2*WI) MRI.[
Previously, a case of intracranial dural arteriovenous fistula (dAVF) treated with open surgery and a spinal dAVF as a cause of symptomatic SS have been reported as the putative cause for SS of CNS.[
CASE DESCRIPTIONS
Case 1
An 88-year-old male referred from outside center with the suspicion of subarachnoid hemorrhage, presented with bilateral upper extremity paresthesias and concern for stroke-like symptoms. The physical examination was unremarkable. His past medical history was significant for the previous stroke with residual right upper extremity paresthesias. A 1.5-tesla MRI revealed susceptibility artifact without associated fluid-attenuated inversion recovery nonsuppression along sulci predominately at the vertex [
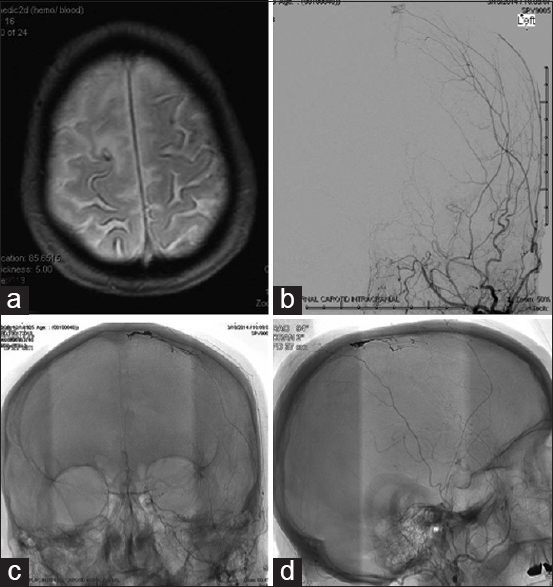
Figure 1
Case 1 - (a) Axial T2-weighted GRE magnetic resonance sequence demonstrating superficial siderosis of the cerebral cortex near the vertex. (b) Cerebral angiography – anteroposterior projection cerebral digital subtraction angiography of the left internal carotid artery injection demonstrating Borden 1 methyl methacrylate to superior sagittal sinus dural arteriovenous fistula. (c and d) Postembolization anteroposterior and lateral nonsubtracted cerebral angiography films showing embolization cast of dural arteriovenous fistula
Case 2
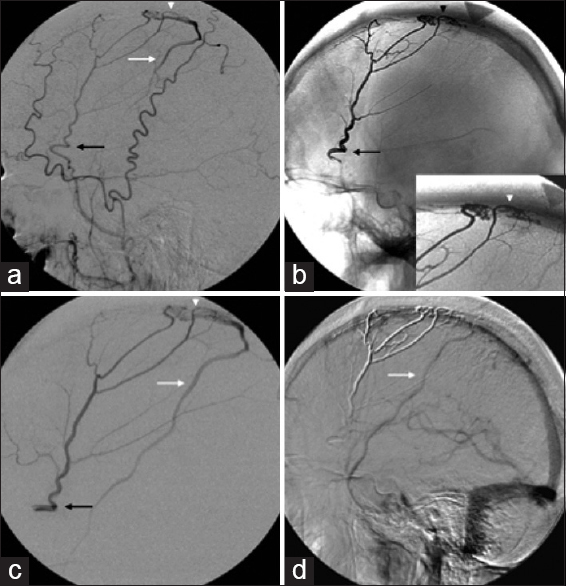
A 73-year-old male presented with a history of progressive cognitive impairment, gait ataxia, and sensorineural hearing loss. MRI of the brain showed classic findings of SS. Conventional angiography showed a Borden III splenic AVF, which was supplied by the right middle meningeal artery and drained into the SSS with cortical venous reflux into the vein of Trolard [Figure
DISCUSSION
Iron and ferritin are found in many types of cells in the brain including neurons, microglia, and oligodendroglia in a normal state.[
SS remains a rare disease with <300 total reported cases since its initial description by Hamill in 1908.[
The most common presentation of SS of CNS is reported to be occult sensorineural hearing loss followed by ataxia/gait imbalance. Corticospinal, cognitive, and olfactory dysfunctions are also frequent, and many patients have been misdiagnosed with diseases such as multiple sclerosis and amyotrophic lateral sclerosis before being diagnosed with SS.[
Dural arteriovenous fistulas (dAVF) represents 10–15% of cerebral vascular malformations and are not commonly associated with SS. There has only been one other case of SS due to an intracranial dAVF described in the literature.[
With the increased availability of MRI and concomitant reductions in cost to obtain these studies, it is presumed that prevalence of SS will increase. The majority of these cases would presumably be asymptomatic and incidental findings, but given the early identification of this potentially debilitating neurologic disease. The combined use of sensitive MR-angiographic techniques such as (four-dimensional time of flight) combined with diagnostic cerebral angiography in a select group of patients will conceivably increase the numbers of patients with SS due to occult intracranial vascular malformations such as dAVF.
While SS due to dAVF should be included in the differential diagnosis of an atypical presentation of neurologic disease, the likelihood of this being the etiology of the patient's disease process is low. An increasing number of reports associate atraumatic cortical SS with cerebral amyloid angiopathy and less commonly with reversible cerebral vasoconstriction syndrome, primary angiitis of the CNS, and reperfusion injury.[
The similar anatomic relationship of both dAVFs reported in the present report as well as the previous case report presents an interesting question about the likely pathogenesis of dAVF-induced SS. The middle meningeal artery supplied the arterial feeders in both lesions as well as in the previous case report, and the drainage pattern was directly into the SSS with cortical venous reflux, whereas it was into transverse sinus in the previous case report. It is plausible that the cortical venous reflux supplied by a high flow venous structure such as the SSS could create sufficient turbulent vascular flow patterns to cause pathologic changes in the walls of the delicate and fragile blood vessels of dAVFs. In addition, similar to the pathologic changes seen in the formation of intracranial arterial aneurysms, it would be possible that changes in the blood vessels themselves might predispose a patient to chronic microhemorrhages resulting in SS.
CONCLUSION
We report the second and third cases of SS due to intracranial dAVF, which were treated with intra-arterial Onyx embolization. As noninvasive imaging techniques become more sensitive and easily obtained, it is crucial to keep in mind occult intracranial vascular malformations such as dAVF as a possible etiology for incidental SS in the appropriate patient population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Anderson NE, Sheffield S, Hope JK. Superficial siderosis of the central nervous system: A late complication of cerebellar tumors. Neurology. 1999. 52: 163-9
2. Cerqueira AC, Nardi AE, Bezerra JM. Superficial siderosis of the central nervous system: An unusual cause of sensorineural hearing loss. Arq Neuropsiquiatr. 2010. 68: 469-71
3. Chandra RV, Leslie-Mazwi TM, Mehta BP, Yoo AJ, Rabinov JD, Pryor JC. Transarterial onyx embolization of cranial dural arteriovenous fistulas: Long-term follow-up. AJNR Am J Neuroradiol. 2014. 35: 1793-7
4. Driver-Dunckley ED, Hoxworth JM, Patel NP, Bosch EP, Goodman BP. Superficial siderosis mimicking amyotrophic lateral sclerosis. J Clin Neuromuscul Dis. 2010. 11: 137-44
5. Fearnley JM, Stevens JM, Rudge P. Superficial siderosis of the central nervous system. Brain. 1995. 118: 1051-66
6. Gonella MC, Fischbein NJ, Lane B, Shuer LM, Greicius MD. Episodic encephalopathy due to an occult spinal vascular malformation complicated by superficial siderosis. Clin Neurol Neurosurg. 2010. 112: 82-4
7. Hamill RC. Report of a case of melanosis of the brain, cord, and meninges. J Nerv Ment Dis. 1908. 35: 594-
8. Hayashida Y, Kakeda S, Hiai Y, Ide S, Ogasawara A, Ooki H. Diagnosis of intracranial hemorrhagic lesions: Comparison between 3D-SWAN (3D T2*-weighted imaging with multi-echo acquisition) and 2D-T2*-weighted imaging. Acta Radiol. 2014. 55: 201-7
9. Hsu WC, Loevner LA, Forman MS, Thaler ER. Superficial siderosis of the CNS associated with multiple cavernous malformations. AJNR Am J Neuroradiol. 1999. 20: 1245-8
10. Hughes JT, Oppenheimer DR. Superficial siderosis of the central nervous system. A report on nine cases with autopsy. Acta Neuropathol. 1969. 13: 56-74
11. Imaizumi T, Chiba M, Honma T, Niwa J. Detection of hemosiderin deposition by T2*-weighted MRI after subarachnoid hemorrhage. Stroke. 2003. 34: 1693-8
12. Jabbarli R, Reinhard M, Niesen WD, Roelz R, Shah M, Kaier K. Predictors and impact of early cerebral infarction after aneurysmal subarachnoid hemorrhage. Eur J Neurol. 2015. 22: 941-7
13. Janss AJ, Galetta SL, Freese A, Raps EC, Curtis MT, Grossman RI. Superficial siderosis of the central nervous system: Magnetic resonance imaging and pathological correlation. Case report. J Neurosurg. 1993. 79: 756-60
14. Koeppen AH, Dentinger MP. Brain hemosiderin and superficial siderosis of the central nervous system. J Neuropathol Exp Neurol. 1988. 47: 249-70
15. Koeppen AH, Dickson AC, Chu RC, Thach RE. The pathogenesis of superficial siderosis of the central nervous system. Ann Neurol. 1993. 34: 646-53
16. Koeppen AH. The history of iron in the brain. J Neurol Sci. 1995. 134: S1-9
17. Kole MK, Steven D, Kirk A, Lownie SP. Superficial siderosis of the central nervous system from a bleeding pseudomeningocele. Case illustration. J Neurosurg. 2004. 100: 718-
18. Kumar N, Cohen-Gadol AA, Wright RA, Miller GM, Piepgras DG, Ahlskog JE. Superficial siderosis. Neurology. 2006. 66: 1144-52
19. Kumar N, Fogelson JL, Morris JM, Pichelmann MA. Superficial siderosis should be included in the differential diagnosis of motor neuron disease. Neurologist. 2012. 18: 139-45
20. Kumar N. Neuroimaging in superficial siderosis: An in-depth look. AJNR Am J Neuroradiol. 2010. 31: 5-14
21. Leussink VI, Flachenecker P, Brechtelsbauer D, Bendszus M, Sliwka U, Gold R. Superficial siderosis of the central nervous system: Pathogenetic heterogeneity and therapeutic approaches. Acta Neurol Scand. 2003. 107: 54-61
22. Li KW, Haroun RI, Clatterbuck RE, Murphy K, Rigamonti D. Superficial siderosis associated with multiple cavernous malformations: Report of three cases. Neurosurgery. 2001. 48: 1147-50
23. Lummel N, Bernau C, Thon N, Bochmann K, Linn J. Prevalence of superficial siderosis following singular, acute aneurysmal subarachnoid hemorrhage. Neuroradiology. 2015. 57: 349-56
24. Lummel N, Wollenweber FA, Demaerel P, Bochmann K, Malik R, Opherk C. Clinical spectrum, underlying etiologies and radiological characteristics of cortical superficial siderosis. J Neurol. 2015. 262: 1455-62
25. Manfredi M, Magni E, Gandolfini M, Beltramello A, Orlandini A, Donati E. Superficial siderosis of the central nervous system and anticoagulant therapy: A case report. Ital J Neurol Sci. 1999. 20: 247-9
26. Martínez-Lizana E, Carmona-Iragui M, Alcolea D, Gómez-Choco M, Vilaplana E, Sánchez-Saudinós MB. Cerebral amyloid angiopathy-related atraumatic convexal subarachnoid hemorrhage: An ARIA before the tsunami. J Cereb Blood Flow Metab. 2015. 35: 710-7
27. McCarron MO, Flynn PA, Owens C, Wallace I, Mirakhur M, Gibson JM. Superficial siderosis of the central nervous system many years after neurosurgical procedures. J Neurol Neurosurg Psychiatry. 2003. 74: 1326-8
28. Mehndiratta P, Mendel TA. Cortical superficial siderosis, APOE genotype, and hemorrhage risk in cerebral amyloid angiopathy. Neurology. 2015. 84: 1190-1
29. Na HK, Park JH, Kim JH, Kim HJ, Kim ST, Werring DJ. Cortical superficial siderosis: A marker of vascular amyloid in patients with cognitive impairment. Neurology. 2015. 84: 849-55
30. Ni J, Auriel E, Jindal J, Ayres A, Schwab KM, Martinez-Ramirez S. The characteristics of superficial siderosis and convexity subarachnoid hemorrhage and clinical relevance in suspected cerebral amyloid angiopathy. Cerebrovasc Dis. 2015. 39: 278-86
31. Nogueira RG, Dabus G, Rabinov JD, Eskey CJ, Ogilvy CS, Hirsch JA. Preliminary experience with onyx embolization for the treatment of intracranial dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2008. 29: 91-7
32. Offenbacher H, Fazekas F, Schmidt R, Kapeller P, Fazekas G. Superficial siderosis of the central nervous system: MRI findings and clinical significance. Neuroradiology. 1996. 38: S51-6
33. Sabat SB. Intraventricular cavernous malformation with superficial siderosis. Arch Neurol. 2010. 67: 638-9
34. Salem A, Krainik A, Helias A, Bouccara D, Gaillard S, Feydy A. MRI findings in a case of a superficial siderosis associated with an ependymoma. J Neuroradiol. 2002. 29: 136-8
35. Satow T, Yamada S, Yagi M, Saiki M. Superficial siderosis of the central nervous system after ventriculoperitoneal shunt. J Neurosurg. 2010. 113: 93-6
36. Savoiardo M, Grisoli M, Pareyson D. Polyradiculopathy in the course of superficial siderosis of the CNS. J Neurol. 2001. 248: 1099-100
37. Shinmei Y, Harada T, Ohashi T, Yoshida K, Moriwaka F, Matsuda H. Trochlear nerve palsy associated with superficial siderosis of the central nervous system. Jpn J Ophthalmol. 1997. 41: 19-22
38. Signorelli F, McLaughlin N, Bojanowski MW. Superficial siderosis as a manifestation of a dural arteriovenous fistula. Can J Neurol Sci. 2011. 38: 367-9
39. Steinberg J, Cohen JE, Gomori JM, Fraifeld S, Moscovici S, Rosenthal G. Superficial siderosis of the central nervous system due to chronic hemorrhage from a giant invasive prolactinoma. J Clin Neurosci. 2013. 20: 1032-4
40. Sydlowski SA, Cevette MJ, Shallop J. Superficial siderosis of the central nervous system: Phenotype and implications for audiology and otology. Otol Neurotol. 2011. 32: 900-8
41. Tacconi L, Marinella T. Superficial siderosis of the central nervous system secondary to a thalamic hamartoma. J Clin Neurosci. 1999. 6: 532-5
42. Tiryaki E, Azzarelli B, Biller J. Superficial siderosis of the central nervous system in a patient with chronic subarachnoid hemorrhage misdiagnosed as multiple sclerosis. J Stroke Cerebrovasc Dis. 2002. 11: 288-9
43. Turner B, Wills AJ. Superficial siderosis associated with anterior horn cell dysfunction. J Neurol Neurosurg Psychiatry. 2002. 72: 274-5
44. Wang K, Xu Z, Xiong G, Benyan L. Superficial siderosis of the central nervous system manifested with seizures. J Clin Neurosci. 2010. 17: 277-8
45. Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004. 5: 863-73
46. Zhao H, Wang J, Lu Z, Wu Q, Lv H, Liu H. Superficial siderosis of the central nervous system induced by a single-episode of traumatic subarachnoid hemorrhage: A study using MRI-enhanced gradient echo T2 star-weighted angiography. PLoS One. 2015. 10: e0116632-