- Department of Neurosurgery, St. George Public Hospital, Kogarah, New South Wales, Australia.
DOI:10.25259/SNI_178_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jiahua Huang, Finn Ghent, Robyn Levingston, Martin Scholsem. Intracranial Ewing Sarcoma – A case report. 30-May-2020;11:134
How to cite this URL: Jiahua Huang, Finn Ghent, Robyn Levingston, Martin Scholsem. Intracranial Ewing Sarcoma – A case report. 30-May-2020;11:134. Available from: https://surgicalneurologyint.com/surgicalint-articles/10061/
Abstract
Background: Intracranial Ewing’s sarcoma (ES) is a rare entity with
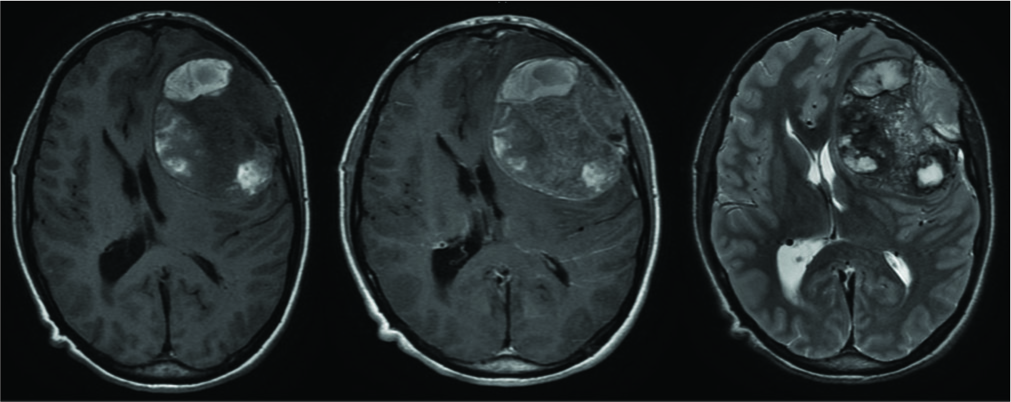
Case Description: We describe a case of intracranial left frontal ES in a 19-year-old patient who presented with change in behavior. Diagnosis was unclear based on radiological findings on MRI and CT alone. MRI brain with contrast demonstrated a large extra-axial ovoid heterogeneously enhancing left frontal convexity mass. The patient underwent gross total resection with adjuvant chemotherapy and radiation. No local or systemic recurrence was found at 12 months postoperatively.
Conclusion: Intracranial ES/pPNET is rare tumor with nonspecific clinical presentation and radiological findings. They are locally invasive. Surgery with adjuvant chemoradiation is the mainstay treatment. Distinction of pPNET and cPNET is important for therapeutic and prognostic purposes.
Keywords: Ewing’s sarcoma, Meningeal tumor, Neuro-oncology, Peripheral primitive neuroectodermal tumor, Soft-tissue tumor
INTRODUCTION
Ewing’s sarcoma and peripheral primitive neuroectodermal tumor (ES/pPNET) belongs to a family of round-cell neuroectodermally derived tumors. They are overlapping entities with the same histological origin but different degree of neuroectodermal differentiation (absent for ES, definite for pPNET).[
We report a rare case of intracranial Ewing sarcoma. The case presentation, imaging findings, operative approach, and postoperative treatment plan are discussed, with a summary of the literature.
CASE DESCRIPTION
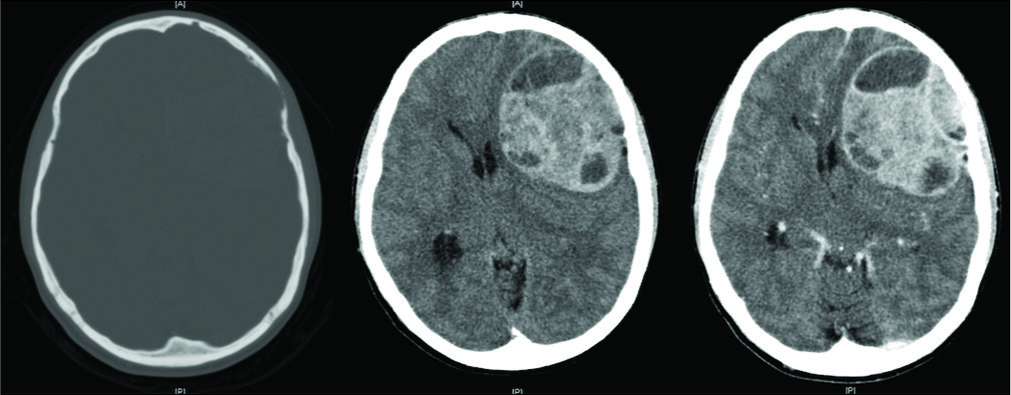
A 19-year-old male with no medical history was referred to our service with approximately 2 weeks of headache and 2 episodes of vomiting. Detailed history from his parents revealed a change in behavior, including apathy and uncharacteristically poor university grades, over the prior 12 months. With the exception of a right pronator drift, his neurological examination was normal. CT brain revealed a large extra-axial heterogeneous mass involving the left frontal and temporal lobes with well-defined lobulated margins and avid enhancement of the high-density areas. The lesion eroded the inner table of the left frontal bone with the outer table almost completely eroded [
Operative management
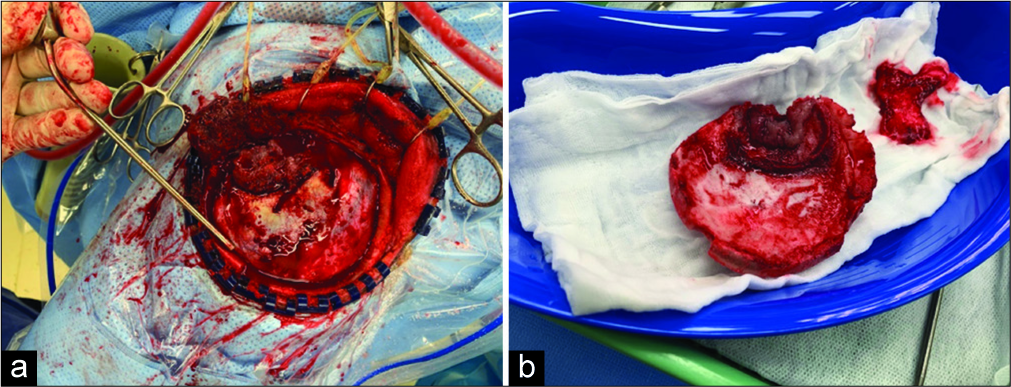
Intraoperatively, the tumor was found to have breached the dura and as suggested by preoperative imaging, had invaded bone [
The patient’s postoperative recovery was uneventful and he was discharged home day 5 after surgery. Postoperative MRI revealed no evidence of residual tumor. PET scanning did not reveal any other lesions. The patient was referred to a local specialist sarcoma unit for adjuvant chemotherapy with VIDE (vincristine, ifosfamide, doxorubicin, and etoposide), to be followed by focal irradiation and second round of chemotherapy with VAI (vincristine, dactinomycin, and ifosfamide).
The patient is now 1-year postsurgery with no clinical or radiological evidence of recurrence or metastatic disease.
Histopathology
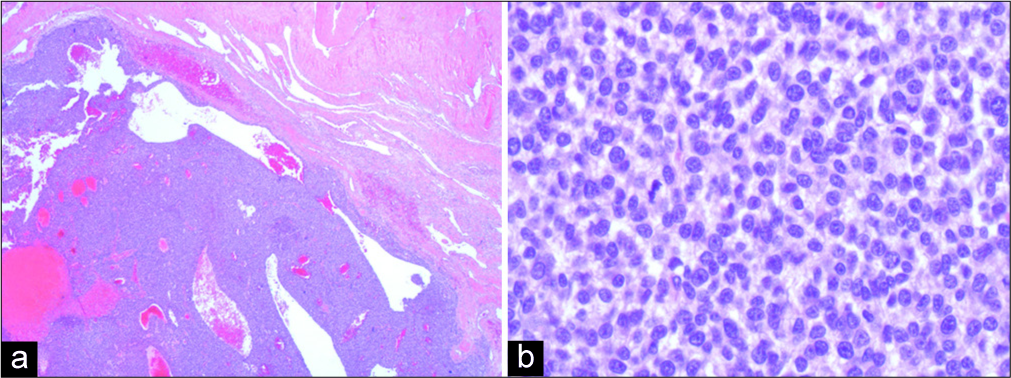
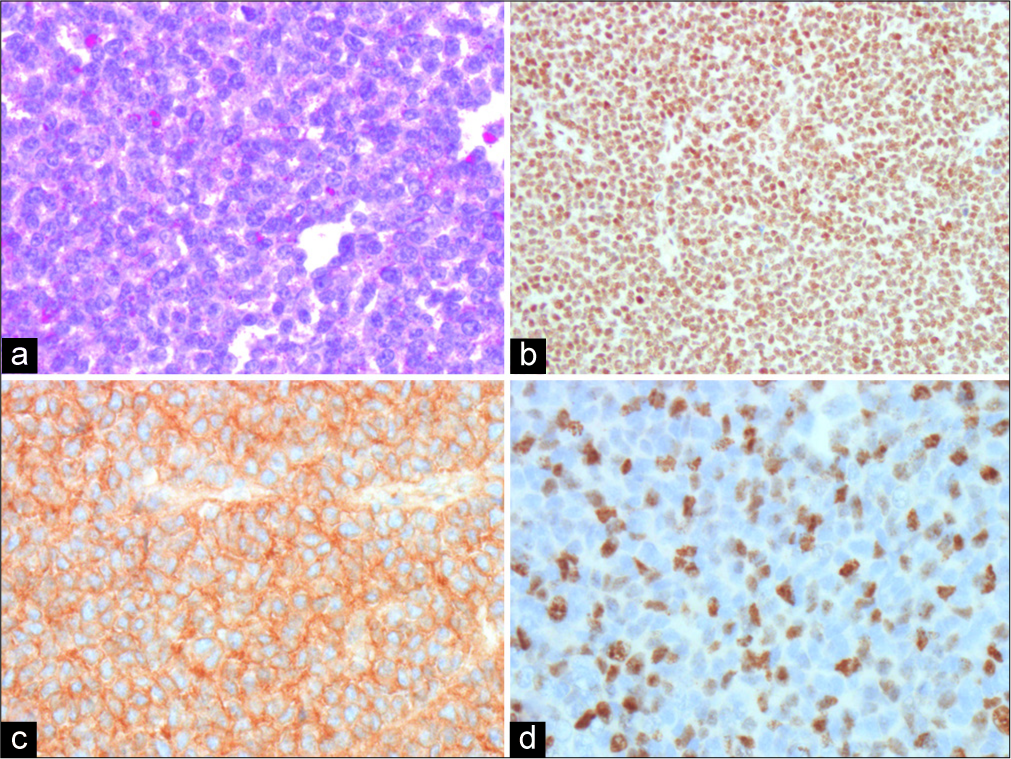
Under light microscopy, the specimen showed a durally based, highly cellular tumor composed of relatively monotonous small blue cells with slightly irregular nuclei and thin rims of cytoplasm [
DISCUSSION
ES/pPNET is neuroectodermal tumors with common histopathological features and a unifying immunophenotypical and genetic profile.[
Radiologically, ES/pPNET often presents as a large, contrast- enhancing, well-circumscribed lesion with dural or bony involvement, mimicking the appearance of a meningioma. They are usually solid but can also have a cystic component. Two patterns of meningeal involvement have been described in the literature.[
The genetic abnormality of ES/pPNET is characterized by the fusion of EWSR1 gene on chromosome 22q12 with a number of other genes, most commonly with the FL11 gene on chromosome 11q24 forming a t(11; 22) (q24; q12) translocation.[
The distinction between ES/cPNET has therapeutic and prognostic importance. cPNETs are embryonal tumors composed of undifferentiated or poorly differentiated neuroepithelial cells with possible divergent differentiation along neuronal, astrocytic, muscular, and melanocytic lines. They are poorly circumscribed and can occur in any region of CNS, but rarely metastases elsewhere. Involvement of cerebrospinal fluid is reported in 10–30% of cases. The treatment of cPNET involves intrathecal methotrexate followed by systemic chemotherapy and radiotherapy. Reported long-term disease-free survival for ES/pPNET ranges from 9 months to 8 years, which is much shorter for cPNET.
There is currently no established treatment protocol for intracranial ES/pPNET. In most cases, the extra-axial location of the tumor enables gross total resection with removal of the dura and bone involved. Most centers follow with adjuvant chemotherapy and focal irradiation.[
CONCLUSION
Intracranial ES/pPNET is rare tumors with nonspecific clinical presentation and radiological features. It is locally aggressive and requires multimodality treatment with surgery and adjuvant chemoradiation therapy. Distinction of pPNET and cPNET is important for therapeutic and prognostic purposes. We have presented a case of frontal ES extending through the cranial vault which was treated with primary resection and adjuvant chemoradiation.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ali AE, Fazl M, Bilbao JM. Primary intracranial leiomyoma: A case report and literature review. Virchows Arch. 2006. 449: 382-4
2. Antunes NL, Lellouch-Tubiana A, Kalifa C, Delattre O, Pierre-Kahn A, Rosenblum MK. Intracranial ewing sarcoma/peripheral primitive neuroectodermal tumor of dural origin with molecular genetic confirmation. J Neurooncol. 2001. 51: 51-6
3. Bano S, Yadav SN, Garga UC. Case report: Intracranial peripheral primitive neuroectodermal tumor-Ewing’s sarcoma of dura with transcalvarial-subgaleal extension: An unusual radiological presentation. Indian J Radiol Imaging. 2009. 19: 305-7
4. Dedeurwaerdere F, Giannini C, Sciot R, Rubin BP, Perilongo G, Borghi L. Primary peripheral PNET/Ewing’s sarcoma of the dura: A clinicopathologic entity distinct from central PNET. Mod Pathol. 2002. 15: 673-8
5. Jain S, Kapoor G. Chemotherapy in Ewing’s sarcoma. Indian J Orthop. 2010. 44: 369-77
6. Janknecht R. EWS-ETS oncoproteins: The linchpins of Ewing tumors. Gene. 2005. 363: 1-14
7. Kumar V, Singh A, Sharma V, Kumar M. Primary intracranial dural-based Ewing sarcoma/peripheral primitive neuroectodermal tumor mimicking a meningioma: A rare tumor with review of literature. Asian J Neurosurg. 2017. 12: 351-7
8. Li Y, Tanaka K, Fan X, Nakatani F, Li X, Nakamura T. Inhibition of the transcriptional function of p53 by EWS-Fli1 chimeric protein in Ewing family tumors. Cancer Lett. 2010. 294: 57-65
9. Ma C, Xu F, Xiao YD, Paudel R, Sun Y, Xiao EH. Magnetic resonance imaging of intracranial hemangiopericytoma and correlation with pathological findings. Oncol Lett. 2014. 8: 2140-4
10. Mobley BC, Roulston D, Shah GV, Bijwaard KE, McKeever PE. Peripheral primitive neuroectodermal tumor/Ewing’s sarcoma of the craniospinal vault: Case reports and review. Hum Pathol. 2006. 37: 845-53
11. Velivela K, Rajesh A, Uppin MS, Purohit AK. Primary intracranial peripheral PNET-a case report and review. Neurol India. 2014. 62: 669-73
12. Yang MJ, Whelan R, Madden J, Levy JM, Kleinschmidt-DeMasters BK, Hankinson TC. Intracranial Ewing sarcoma: Four pediatric examples. Childs Nerv Syst. 2018. 34: 441-8