- Department of Neurosurgery, Hartford Hospital, Hartford, Connecticut, United States,
- Department of Infectious Disease, Hartford Hospital, Hartford, Connecticut, United States,
- Department of Neurointensive Care, Hartford Hospital, Hartford, Connecticut, United States.
Correspondence Address:
Nicholas Zacharewski, Department of Neurosurgery, Hartford Hospital, Hartford, Connecticut, United States.
DOI:10.25259/SNI_116_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kent J. Kilbourn1, Jaquise Green1, Nicholas Zacharewski1, Joseph Aferzon1, Michael Lawlor2, Matthew Jaffa3. Intracranial fungal Cladophialophora bantiana infection in a nonimmunocompromised patient: A case report and review of the literature. 22-Apr-2022;13:165
How to cite this URL: Kent J. Kilbourn1, Jaquise Green1, Nicholas Zacharewski1, Joseph Aferzon1, Michael Lawlor2, Matthew Jaffa3. Intracranial fungal Cladophialophora bantiana infection in a nonimmunocompromised patient: A case report and review of the literature. 22-Apr-2022;13:165. Available from: https://surgicalneurologyint.com/surgicalint-articles/11550/
Abstract
Background: Cladophialophora bantiana is a dematiaceous fungus that rarely infects the central nervous system (CNS). It is associated with a mortality rate of over 70% despite treatment.
Case Description: An 81-year-old female with a remote history of renal cell carcinoma presented with progressive headache and an expressive aphasia for 3 days. Computed tomography imaging revealed a left frontotemporal mass with surrounding vasogenic edema. A left frontotemporal craniotomy was performed and cultures revealed C. bantiana. The initial management with IV voriconazole was unsuccessful and the patient had a recurrence of the cranial infection and developed pulmonary abscesses. Following the addition of oral flucytosine, the patient showed a significant improvement with a complete radiographic resolution of both the cranial and pulmonary lesions.
Conclusion: C. bantiana involving the CNS is a rare and often fatal disease. Surgical management along with standard antifungal treatment may not provide definitive therapy. The addition of flucytosine to IV voriconazole resulted in a positive outcome for this patient who is alive, living independently 1 year from the original diagnosis. In this rare fungal infection, standard antifungal treatment may not provide adequate coverage and the utilization of additional therapy may be required.
Keywords: Central nervous system, Cladophialophora bantiana, Cranial abscess, Flucytosine, Fungal, Infection
INTRODUCTION
Dematiaceous fungi are identified by the melanin-containing hyphae and conidia that provide the darkly pigmented color for which they are named.[
Various medications have been used for the treatment of CNS C. bantiana including the broad-spectrum antifungal amphotericin B. Treatment failure and risks of serious side effects have changed the treatment paradigm to the use of newer azole drugs which offer important advantages including the availability for oral treatment and low risk for nephrotoxicity. Infections of the CNS pose multiple challenges including determining the duration of treatment and the need for permeating the blood–brain barrier. In resistant cases, the need for combination treatment may be necessary. The antimetabolite drug flucytosine, once used as a stand-alone therapy for systemic fungal infections, has been used with amphotericin B in reported cases, although the outcomes have been mixed. Here, we report the successful use of concomitant treatment with flucytosine and voriconazole in a patient with disseminated black mold infection.
CASE PRESENTATION
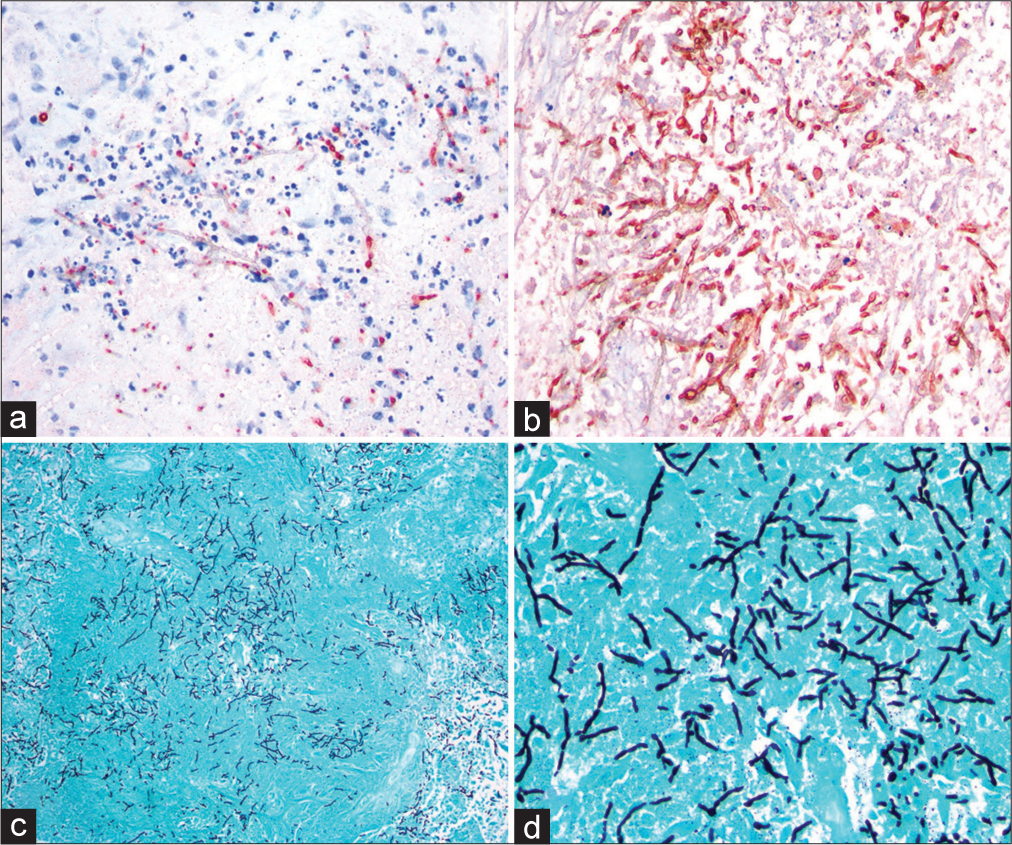
An 81-year-old Caucasian female with a remote history of renal cell carcinoma, hypertension, and pulmonary nodules presented to the hospital with 3 days of headaches and confusion. On examination, the patient was found to have an expressive aphasia. Computed tomography (CT) of the head demonstrated a heterogeneously enhancing left temporal mass with surrounding edema concerning for a primary neoplasm. The patient was then transferred to our institution for neurosurgical consultation. Brain magnetic resonance imaging (MRI) with contrast was completed revealing a heterogeneously enhancing mass involving the left temporal lobe with extensive vasogenic edema raising concern for glial neoplasm [
Six weeks following initial operative management, the patient was readmitted with worsening confusion and repeat imaging demonstrated a recurrent abscess with significant edema and midline shift [
Figure 3:
(a) Six-week follow-up contrast-enhanced axial T1-weighted MRI showing disease progression with worsening edema and mass effect. (b) One-week postoperative redo craniotomy T1-weighted contrast-enhanced T1 axial MRI showing worsening edema with a new cystic collection with mass effect and developing communicating hydrocephalus.
Remarkably, the patient had a significant improvement at her 6-week follow-up visit and an MRI of the brain showed decreasing size of the temporal lesion. A 8-month follow- up showed complete resolution of the left temporal lesion [
DISCUSSION
C. bantiana carries a high mortality when involving the CNS despite aggressive surgical and pharmacologic treatment.[
Four organisms are primarily responsible for cerebral phaeohyphomycosis: Cladosporium trichoides, Xylohypha bantiana, Cladosporium bantianum, and C. bantiana. These four organisms fall under the mycological category of C. bantiana.[
CNS invasive C. bantiana is relatively uncommon and carries a nearly 70% mortality rate despite surgery resection and use of systemic antifungal treatments.[
Amphotericin B has long been considered the mainstay of treatment and more recently, in combination with flucytosine.[
Flucytosine is a pro-drug of 5-fluorouracil (5-FU) that acts as a purine and pyrimidine uptake inhibitor and is used as a fungistatic agent in conjunction with an antifungal such as amphotericin B for the treatment of cryptococcal pneumonia or Candida sepsis in immunocompromised patients. Within the fungal organism, flucytosine metabolizes to flucytosine 5-FU which inhibits both DNA and RNA synthesis. The medication has been around for more than a half of a century; however, increased resistance and the risk of nephrotoxicity and bone marrow suppression have shifted therapy toward newer medications.
Our patient’s initial treatment consisted of a complete surgical resection of the mass followed by systemic amphotericin B. When the patient returned with a recurrence of the abscess, the treatment was shifted to IV voriconazole. While amphotericin B remains the gold standard for the treatment of invasive fungal infections, its efficacy is limited, with response rates ranging widely from 10% to 80%.[
Historically, systemic treatment for C. bantiana has included amphotericin B and more recently – azoles such as voriconazole, itraconazole, ketoconazole, and fluconazole see [
As cases of phaeohyphomycosis continue to rise the need for more efficacious therapies must be identified as treatment options. Stand-alone treatment with a single agent antifungal regimen may not be sufficient. This rare case highlights the use of a multifaceted approach, including the use of older generation medications with good outcome.
CONCLUSION
We present a rare case of a fungal brain abscess caused by C. bantiana (black mold). Our patient’s lesion initially originated in the left frontal-temporal region and rapidly disseminated throughout the entire right lung, including a lesion to the right upper lung. Complete surgical resection followed by combination antifungal medications resulted in clinical and radiographic resolution at 1-year follow-up.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Badali H, Chander J, Bansal S, Aher A, Borkar SS, Meis JF. First autochthonous case of Rhinocladiella mackenziei cerebral abscess outside the Middle East. J Clin Microbiol. 2010. 48: 646-9
2. Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, Arendrup MC. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: Diseases caused by black fungi. Clin Microbiol Infect. 2014. 20: 47-75
3. Deng S, Pan W, Liao W, de Hoog GS, van den Ende AH, Vitale RG. Combination of amphotericin B and flucytosine against neurotropic species of melanized fungi causing primary cerebral phaeohyphomycosis. Antimicrob Agents Chemother. 2016. 60: 2346-51
4. Fica A, Diaz MC, Luppi M, Olivares R, Saez L, Baboor M. Unsuccessful treatment with voriconazole of a brain abscess due to Cladophialophora bantiana. Scand J Infect Dis. 2003. 35: 892-3
5. Harrison JG, Beltran LP, Buerkle CA, Cook D, Gardner DR, Parchman TL. A suite of rare microbes interacts with a dominant, heritable, fungal endophyte to influence plant trait expression. ISME J. 2021. 15: 2763-78
6. Jabeen K, Farooqi J, Zafar A, Jamil B, Mahmood SF, Ali F. Rhinocladiella mackenziei as an emerging cause of cerebral phaeohyphomycosis in Pakistan: A case series. Clin Infect Dis. 2011. 52: 213-7
7. Kantarcioglu AS, Guarro J, de Hoog GS, Apaydin H, Kiraz N, Balkan II. A case of central nervous system infection due to Cladophialophora bantiana. Rev Iberoam Micol. 2016. 33: 237-41
8. Kantarcioglu AS, Guarro J, De Hoog S, Apaydin H, Kiraz N. An updated comprehensive systematic review of Cladophialophora bantiana and analysis of epidemiology, clinical characteristics, and outcome of cerebral cases. Med Mycol. 2017. 55: 579-604
9. Podnos YD, Anastasio P, De La Maza L, Kim RB. Cerebral phaeohyphomycosis caused by Ramichloridium obovoideum (Ramichloridium mackenziei): Case report. Neurosurgery. 1999. 45: 372-5
10. Revankar SG. Cladophialophora bantiana brain abscess in an immunocompetent patient. Can J Infect Dis Med Microbiol. 2011. 22: 149-50
11. Tiphine M, Letscher-Bru V, Herbrecht R. Amphotericin B and its new formulations: Pharmacologic characteristics, clinical efficacy, and tolerability. Transpla Infect Dis. 1999. 1: 273-83
12. Vermes A. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000. 46: 171-9