- Department of Neurosurgery, Iwate Medical University, Yahaba, Iwate, Japan.
Correspondence Address:
Yoshitaka Kubo, Department of Neurosurgery, Iwate Medical University, Yahaba, Iwate, Japan.
DOI:10.25259/SNI_1223_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takahiro Koji, Yoshitaka Kubo, Yoshiyasu Matsumoto, Yosuke Akamatsu, Kohei Chida, Hiroshi Kashimura, Kuniaki Ogasawara. Intracranial hemorrhage associated with direct oral anticoagulant after clipping for an unruptured cerebral aneurysm: A report of two cases. 25-Mar-2022;13:104
How to cite this URL: Takahiro Koji, Yoshitaka Kubo, Yoshiyasu Matsumoto, Yosuke Akamatsu, Kohei Chida, Hiroshi Kashimura, Kuniaki Ogasawara. Intracranial hemorrhage associated with direct oral anticoagulant after clipping for an unruptured cerebral aneurysm: A report of two cases. 25-Mar-2022;13:104. Available from: https://surgicalneurologyint.com/surgicalint-articles/11486/
Abstract
Background: Two cases of patients who developed intracranial hemorrhage associated with direct oral anticoagulant (DOAC) use after clipping of an unruptured cerebral aneurysm (uAN) are presented. These cases will help neurosurgeons assess the risks of patients with atrial fibrillation or deep venous thrombosis receiving DOACs who require craniotomy.
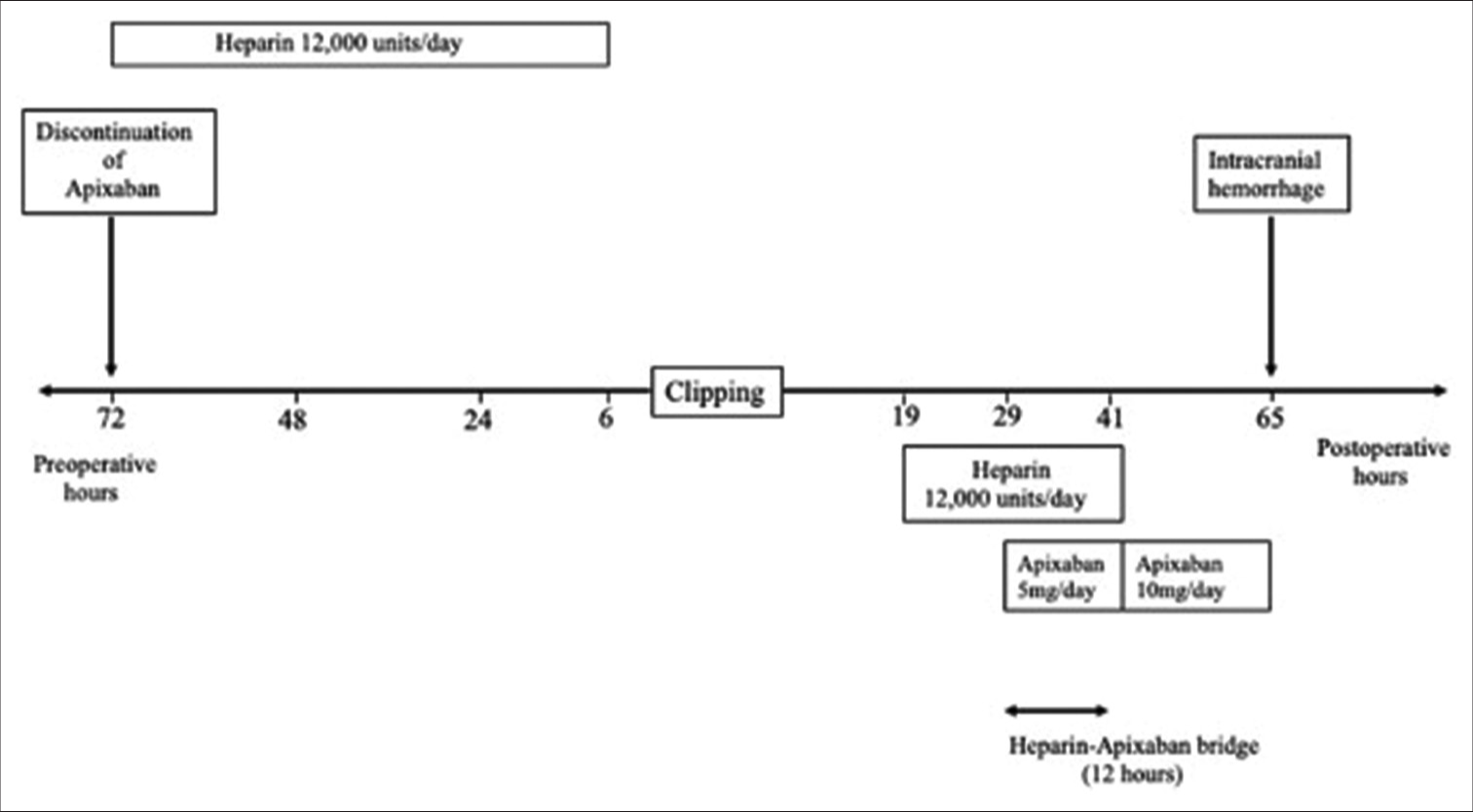
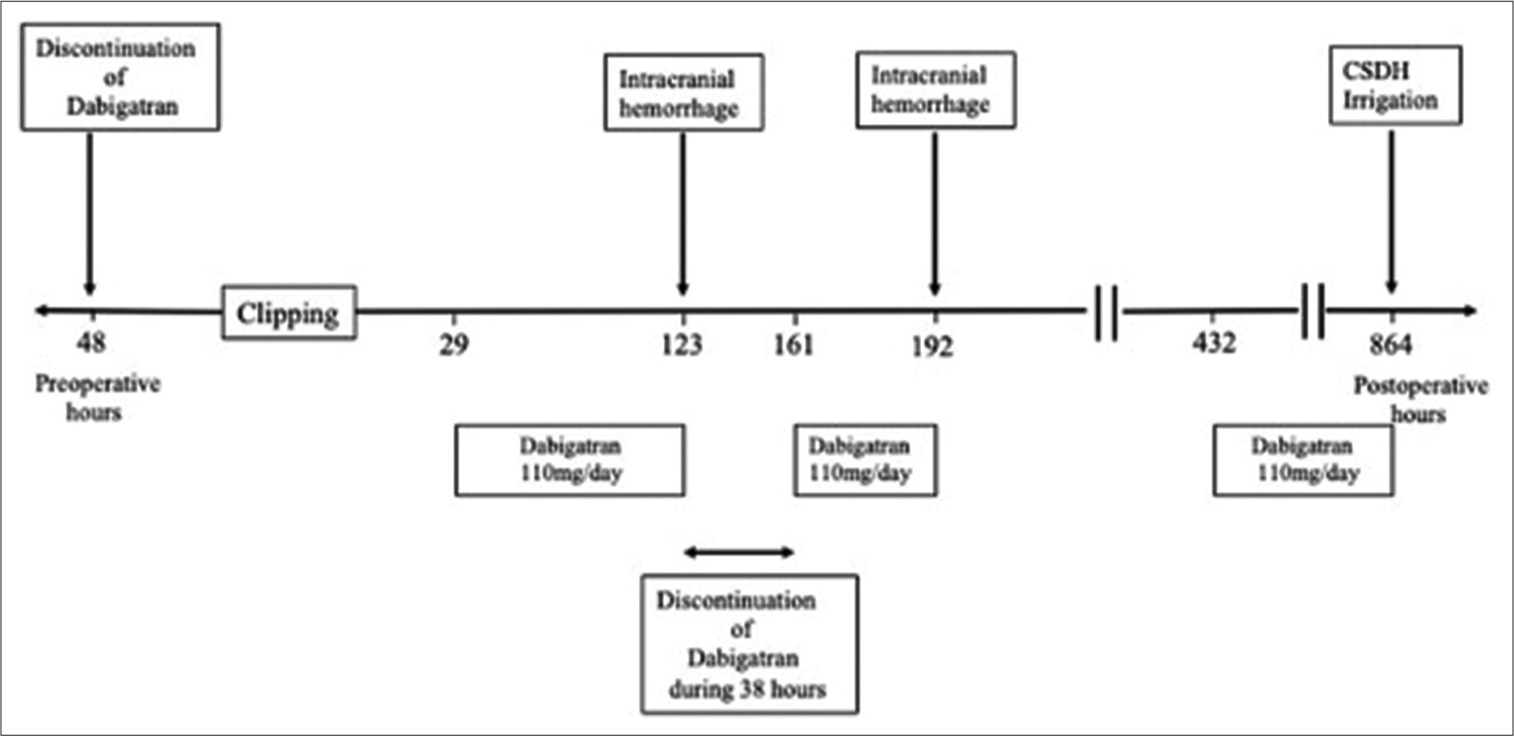
Case Description: Case 1 was a 65-year-old man on apixaban 10 mg/day who underwent clipping for a left middle cerebral artery uAN. Apixaban was discontinued 72 h before surgery. During surgery, a thin and pial artery bled slightly at 1 point of the frontal lobe, and hemostasis was easily achieved. Computed tomography (CT) 19 h after surgery showed no evidence of intracranial hemorrhage. He was treated with a heparin-apixaban bridge from 29 h to 41 h after surgery. CT showed a left subarachnoid hematoma 24 h later. Case 2 was a 73-year-old woman on dabigatran 110 mg/day who underwent clipping for a right MCA uAN. Dabigatran was discontinued 48 h before surgery. During surgery, a thin and pial artery bled slightly at 2 points of the temporal lobe, and hemostasis was easily achieved. CT 19 h after surgery showed no evidence of intracranial hemorrhage. Dabigatran (110 mg/day) was restarted 29 h after surgery. CT then showed a right subarachnoid hematoma 94 h later, and dabigatran was discontinued, and it was then restarted 38 h later. However, 31 h later, CT showed an additional slight subarachnoid hemorrhage. Finally, she developed a right chronic subdural hematoma.
Conclusion: In patients undergoing neurosurgical procedures, discontinuation of DOACs should be individualized based on neurosurgical bleeding risk and patient renal function. Restarting of DOACs could be considered after at least 48 h when hemostasis has been achieved. Bridging of DOACs cannot be recommended.
Keywords: Cerebral aneurysm, Clipping, Direct oral anticoagulant, Intracranial hemorrhage
INTRODUCTION
Several previous studies including autopsy reports have demonstrated that the formation and growth of unruptured cerebral aneurysms that might result in subarachnoid hemorrhage increase with advancing age.[
Two cases of patients who underwent craniotomy for surgical clipping of unruptured cerebral aneurysms and developed intracranial hemorrhage associated with DOACs are presented. These cases will help neurosurgeons assess the risks of patients with Af or deep venous thrombosis receiving DOACs who require craniotomy.
CASE DESCRIPTION
Case 1
A 65-year-old man was on apixaban 10 mg/day as a DOAC for embolic cerebral infarction in the left occipital lobe and cerebellum associated with paroxysmal nonvalvular Af. He has a CHADS2-VASc score[
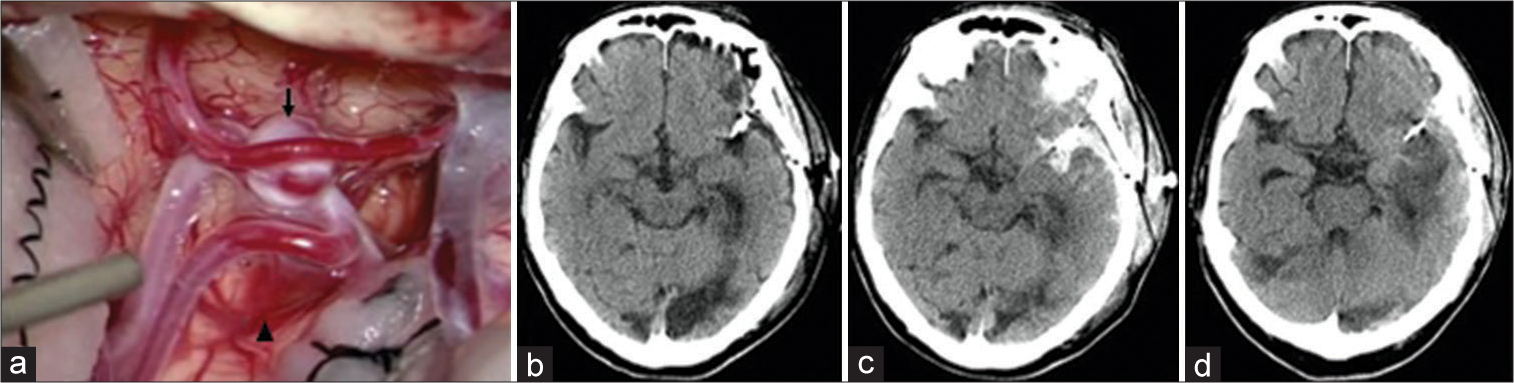
Figure 1:
Case 1 (a) During surgical clipping for an aneurysm (arrow) of the left middle cerebral artery, a thin and pial artery bleeds slightly at 1 point of the left frontal lobe (arrowheads), and hemostasis is easily achieved using cotton. (b) Computed tomography (CT) 19 h after surgery shows no evidence of intracranial hemorrhage. (c) CT 36 h after restarting apixaban (24 h after discontinuing the heparin-DOAC bridge) shows subarachnoid hemorrhage in the left Sylvian fissure. (d) CT 240 h after surgery shows no additional increase of the hematoma.
Case 2
A 73-year-old woman was on dabigatran 110 mg/day as a DOAC for embolic cerebral infarction in the right frontal lobe with paroxysmal nonvalvular Af. She had a CHADS2-VASc score[
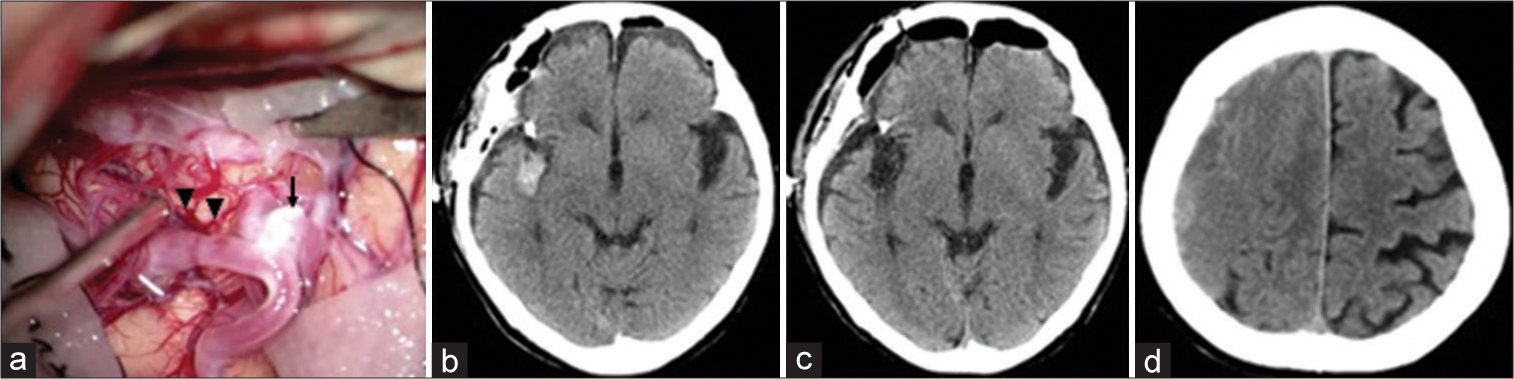
Figure 3:
Case 2 (a) During surgical clipping for an aneurysm (arrow) of the right middle cerebral artery, a thin and pial artery bleeds slightly at 2 points of the right temporal lobe (arrowheads), and hemostasis is easily achieved using cotton. (b) Computed tomography (CT) 19 h after surgery shows no evidence of intracranial hemorrhage. (c) CT 94 h after restarting dabigatran shows subarachnoid hemorrhage in the right Sylvian fissure. (d) CT 864 h after surgery shows a right chronic subdural hematoma.
DISCUSSION
Although the present two patients underwent gentle surgical clipping for unruptured cerebral aneurysms, intracranial hemorrhage developed. According to a meta-analysis[
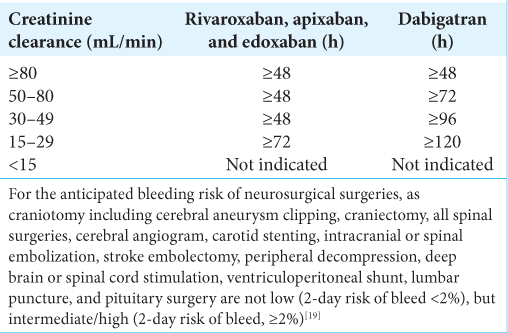
Therefore, it is important to determine when to discontinue and restart DOACs to avoid bleeding in invasive neurosurgical procedures. According to the previous reports,[
CONCLUSION
Two cases of patients who developed intracranial hemorrhage associated with DOAC use after surgical clipping for unruptured cerebral aneurysms were presented. These cases will help neurosurgeons assess risks in patients on DOACs requiring craniotomy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work was partly supported by a Grant-in-Aid for Strategic Medical Science Research (S1491001) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, a Research Grant of Japanese National Hospital Organization Kamaishi Hospital and a grant from JSPS KAKENHI (21K09158).
References
1. Algra AM, Lindgren A, Vergouwen MD, Greving JP, van der Schaaf IC, van Doormaal TP. Procedural clinical complications, case-fatality risks, and risk factors in endovascular and neurosurgical treatment of unruptured intracranial aneurysms: A systematic review and meta-analysis. JAMA Neurol. 2019. 76: 282-93
2. Aronow WS, Ahn C, Gutstein H. Prevalence of atrial fibrillation and association of atrial fibrillation with prior and new thromboembolic stroke in older patients. J Am Geriatr Soc. 1996. 44: 521-3
3. Barnes GD, Mouland E. Peri-procedural management of oral anticoagulants in the DOAC era. Prog Cardiovasc Dis. 2018. 60: 600-6
4. Basali A, Mascha E, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology. 2000. 93: 48-54
5. Beyer-Westendorf J, Gelbricht V, Forster K, Ebertz F, Köhler C, Werth S. Peri-interventional management of novel oral anticoagulants in daily care: Results from the prospective Dresden NOAC registry. Eur Heart J. 2014. 35: 1888-96
6. Croci DM, Kamenova M, Guzman R, Mariani L, Soleman J. Novel oral anticoagulants in patients undergoing cranial surgery. World Neurosurg. 2017. 105: 841-8
7. Doherty JU, Gluckman TJ, Hucker WJ, Januzzi JL, Ortel TL, Saxonhouse SJ. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: A report of the American college of cardiology clinical expert consensus document task force. J Am Coll Cardiol. 2017. 69: 871-98
8. Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015. 27;373: 823-33
9. Healey JS, Eikelboom J, Douketis J, Wallentin L, Oldgren J, Yang S. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: Results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012. 126: 343-8
10. Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W. Updated European heart rhythm association practical guide on the use of non-Vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015. 17: 1467-507
11. Hishikawa T, Date I, Tokunaga K, Tominari S, Nozaki K, Shiokawa Y. Risk of rupture of unruptured cerebral aneurysms in elderly patients. Neurology. 2015. 85: 1879-85
12. Iwamoto H, Kiyohara Y, Fujishima M, Kato I, Nakayama K, Sueishi K. Prevalence of intracranial saccular aneurysms in a Japanese community based on a consecutive autopsy series during a 30-year observation period. Hisayama study. Stroke. 1999. 30: 1390-5
13. Kang JG, Ahn HJ, Kim GS, Hahm TS, Lee JJ, Gwak MS. The hemostatic profiles of patients with Type O and non-O blood after acute normovolemic hemodilution with 6% hydroxyethyl starch (130/0.4). Anesth Analg. 2006. 103: 1543-8
14. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016. 37: 2893-962
15. Kubo Y, Koji T, Kashimura H, Otawara Y, Ogawa A, Ogasawara K. Female sex as a risk factor for the growth of asymptomatic unruptured cerebral saccular aneurysms in elderly patients. J Neurosurg. 2014. 121: 599-604
16. Moeller A, Weippert-Kretschmer M, Prinz H, Kretschmer V. Influence of ABO blood groups on primary hemostasis. Transfusion. 2001. 41: 56-60
17. Ohira T, Cushman M, Tsai MY, Zhang Y, Heckbert SR, Zakai NA. ABO blood group, other risk factors and incidence of venous thromboembolism: The longitudinal investigation of thromboembolism etiology (LITE). J Thromb Haemost. 2007. 5: 1455-61
18. Seifman MA, Lewis PM, Rosenfeld JV, Hwang PY. Postoperative intracranial haemorrhage: A review. Neurosurg Rev. 2011. 34: 393-407
19. Verma A, Ha AC, Rutka JT, Verma S. What surgeons should know about non-Vitamin K oral anticoagulants: A review. JAMA Surg. 2018. 153: 577-85
20. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham study. Stroke. 1991. 22: 983-8
21. Zakai NA, Judd SE, Alexander K, McClure LA, Kissela BM, Howard G. ABO blood type and stroke risk: The reasons for geographic and racial differences in stroke study. J Thromb Haemost. 2014. 12: 564-70