- Department of Neurosurgery, Kohka Public Hospital, Kohka,
- Department of Surgery, Japanese Red Cross Nagahama Hospital, Nagahama, Japan,
- Department of Cardiology, Japanese Red Cross Nagahama Hospital, Nagahama, Japan,
- Department of Neurosurgery, Japanese Red Cross Nagahama Hospital, Nagahama, Japan.
Correspondence Address:
Sayaka Ito, Department of Neurosurgery, Kohka Public Hospital, Kohka, Japan.
DOI:10.25259/SNI_324_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sayaka Ito1, Masanobu Taniguchi2, Yuki Uemura3, Kazushi Higuchi4. Intracranial venous reflux without the central venous occlusive disease in a patient receiving hemodialysis through brachio-brachial arteriovenous fistula: A case report. 06-May-2022;13:190
How to cite this URL: Sayaka Ito1, Masanobu Taniguchi2, Yuki Uemura3, Kazushi Higuchi4. Intracranial venous reflux without the central venous occlusive disease in a patient receiving hemodialysis through brachio-brachial arteriovenous fistula: A case report. 06-May-2022;13:190. Available from: https://surgicalneurologyint.com/surgicalint-articles/11582/
Abstract
Background: Upper-limb arteriovenous fistula as a hemodialysis access among patients with end-stage renal disease (ESRD) has become a preferred type of vascular access. However, complications involving the central nervous system may occur. There have been no reported cases of internal jugular vein (IJV) regurgitation without central venous occlusive diseases (CVODs).We describe the case of a patient on HD who presented with symptomatic IJV regurgitation without CVODs.
Case Description: An 83-year-old man with ESRD receiving HD through a left upper-limb AVF presented with impaired consciousness and seizures. After recovery from unconsciousness, he became alert with cognitive impairment. The left subclavian arteriography revealed early filling of the left subclavian vein due to the AVF on the left brachium, with retrograde high-flow venous reflux to the left IJV, sigmoid and transverse sinuses, with the left central veins patent. All cerebral venous drainage procedures were dependent on the right IJV. The left internal carotid arteriography showed venous congestion of the left hemisphere. The flow of the left brachial artery was measured extremely high. Under compression of the left brachial artery to reduce the flow, the regurgitation persisted. With the findings that all cerebral venous return were in the right IJV, sacrificing the left IJV was thought to be acceptable. Left IJV ligation was performed, and the patient’s cognitive function improved.
Conclusion: The short-term outcome after IJV ligation may be positive in the patient who was confirmed to have a normal cerebral venous return route independent of the refluxed IJV.
Keywords: Central venous disease, End-stage renal disease, Internal jugular vein valve, Internal jugular vein, Pseudophebitic pattern
INTRODUCTION
The number of patients with end-stage renal disease (ESRD) undergoing hemodialysis (HD) has been increasing worldwide. HD access remains a crucial aspect of patient management, especially concerning quality of life without complications. Patients undergoing HD require vascular access through an arteriovenous fistula (AVF) or arteriovenous graft to obtain an adequate blood flow rate to perform HD if they are independent of a central venous catheter.[
Complications involving the central nervous system may occur and there have been many reports of internal jugular vein (IJV) regurgitation in HD patients with the central venous occlusive diseases (CVODs).[
Here, we describe the case of a patient on HD who presented with symptomatic IJV regurgitation without CVODs. We also discuss the etiology of IJV regurgitation and treatment of cerebral venous hypertension due to IJV regurgitation.
The patient provided informed consent to publish this case report and his identity was protected.
CASE DESCRIPTION
In this report, we present the case of an 83-year-old right-handed male patient with ESRD. The patient, who had been receiving HD for 1.5 years through a left upper-limb AVF, was brought to our hospital on the day following his last HD due to impaired consciousness and status epilepticus. The patient’s left arm was not edematous. His medical history included chronic renal failure, ischemic coronary artery disease, and post craniotomy state from the total removal of meningioma on the left frontal convexity 20 years before admission. He independently drove himself to receive biweekly HD treatment before admission. After controlling his seizures using anticonvulsants, he underwent imaging studies, including brain computed tomography and magnetic resonance (MR) imaging/angiography. MR angiography showed a high signal in the right transverse sinus, sigmoid sinus, and jugular bulb [
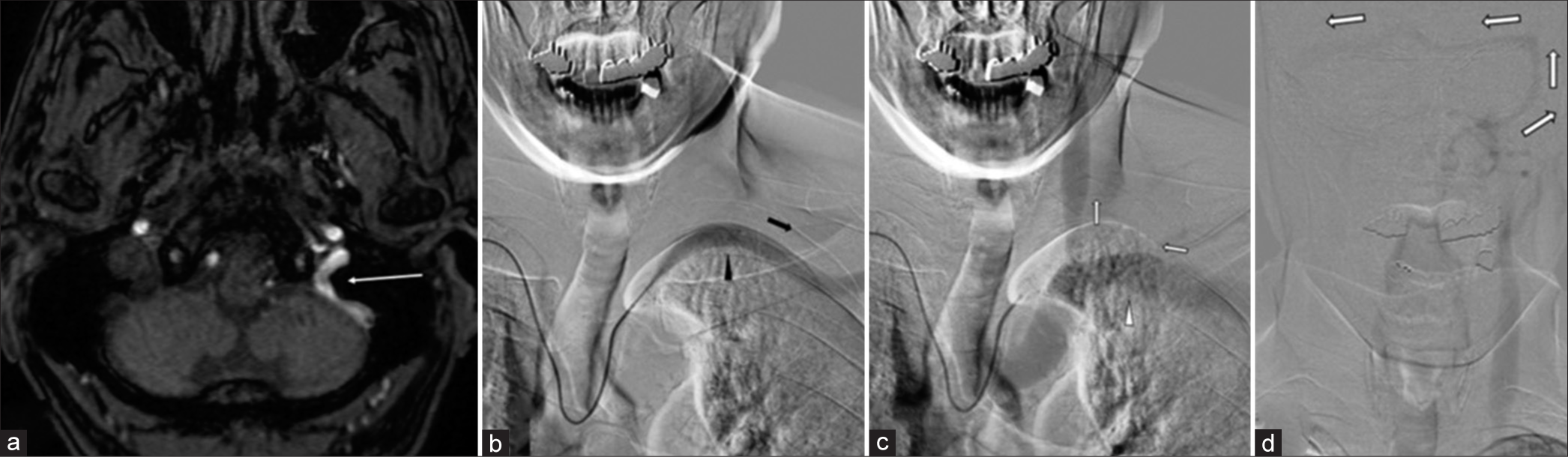
Figure 1:
MR angiography at admission showing a high signal in the left jugular bulb (arrow in a). The left subclavian arteriography showing the left subclavian artery (black arrowhead of b) flowing distally (black arrow in b), the left subclavian vein (white arrowhead in c) flowing into the left brachiocephalic vein with the retrograde flow into the left internal jugular vein, the left sigmoid sinus, and transverse sinus, inserting to the confluence, and draining through the right transverse sinus (white arrows in c and d).
The patient experienced aphasia and right hemiparesis for 2 days after gaining consciousness. The Mini-Mental State Examination (MMSE) was used to evaluate the patients’ cognitive function immediately after he became alert, and a score of 16 points was measured, indicating moderate cognitive impairment. Digital subtraction angiography (DSA) was performed and left subclavian arteriography (AG) revealed early filling of the left subclavian vein due to the AVF on the left brachium, with retrograde high-flow venous reflux to the left IJV, sigmoid sinus, and transverse sinus, with the left brachiocephalic vein and superior vena cava patent. The retrograde venous flow was inserted into the confluence and drained through the right IJV [
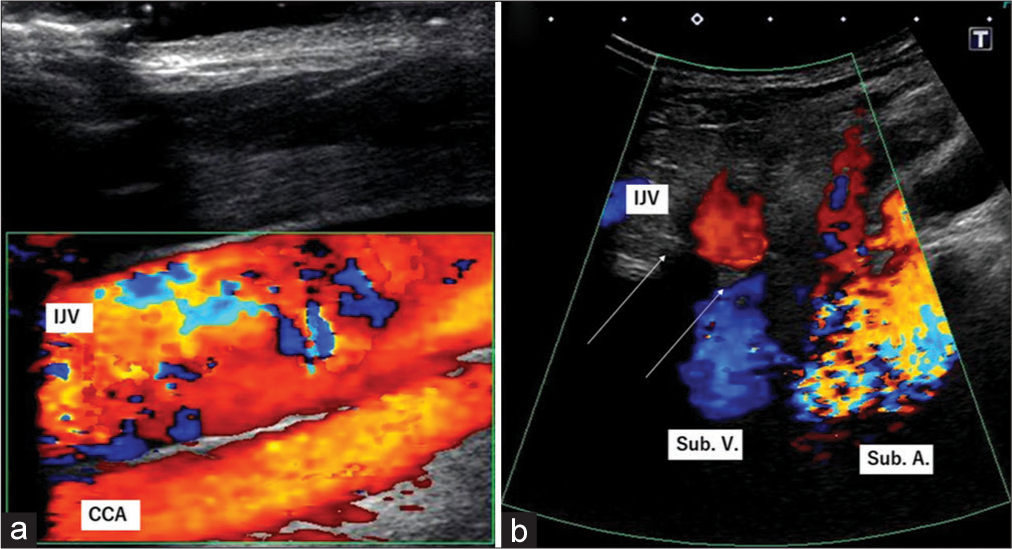
Further examinations were performed using duplex ultrasonography (US). The flow of the left brachial artery was measured at 3150 ml/min, which indicated that the extremely high flow was produced by the brachio-brachial AVF. The brachial vein bifurcated into two major trunks on the proximal side of the anastomosis. Retrograde venous flow to the left IJV from the left subclavian vein was revealed using US [
Vascular surgeons along with cardiovascular physicians and neurosurgeons discussed the treatment strategy. A flow reduction procedure of the left brachio-brachial AVF was suggested for long-term cardiac outcome under HD, with prevention of heart failure by a high-flow shunt; however, this procedure was not sufficient to terminate the left IJV regurgitation caused by the high-flow AVF. There was no need for an endovascular approach to the central veins because they were patent. With the finding that all cerebral venous return was in the right IJV, the sacrificing the left IJV was thought to be acceptable. The first priority was to terminate IJV regurgitation to urgently normalize intracranial pressure, followed by flow-reduction surgery of the AVF for a long-term systemic prognosis.
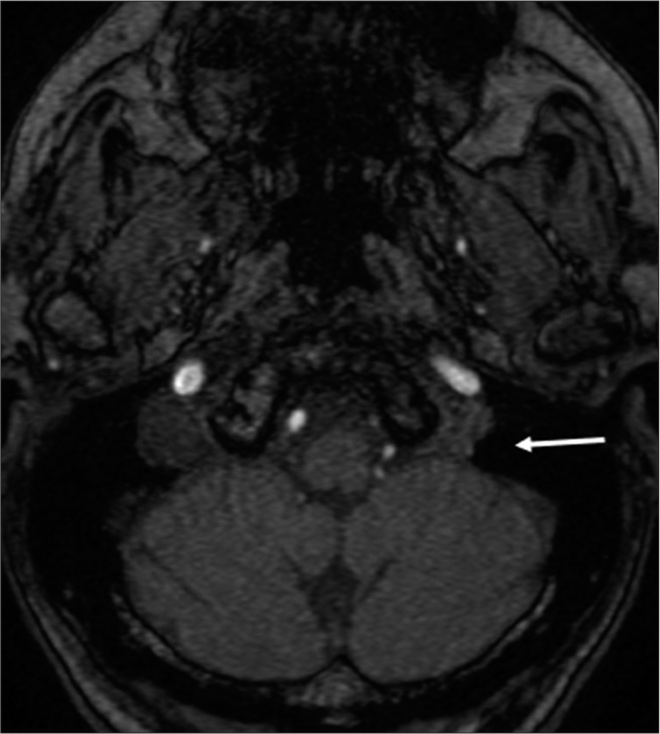
Thus, the left IJV ligation was performed under general anesthesia. Videoangiography using indocyanine green performed before ligation revealed retrograde venous flow in the left IJV. Videoangiography performed after ligation showed no flow into the IJV. Immediately, after surgery, the patient’s MMSE score improved to 24 points and persisted until discharge. Post-operative US showed no regurgitation to the ligated IJV, with high flow of the brachial artery. Brain MR angiography did not show high-intensity signals in any of the intracranial dural sinuses [
DISCUSSION
The well-known adverse effects of the upper-limb AVF for HD include left ventricular hypertrophy, high-output cardiac failure, exacerbation of coronary ischemia, and central vein stenosis.[
In most cases, the IJV valve, which is reportedly the only venous valve between the right atrium of the heart and the brain, has been thought to play an important role in preventing regurgitation from the subclavian vein to the IJV, especially when intrathoracic pressure is increased. However, acquired or congenital IJV valve incompetence may impair cerebral venous return under the Valsalva maneuver resulting from IJV regurgitation affecting cerebral circulation.[
Cases of intracranial venous reflux associated with CVODs in HD patients with neurological deficits have been reported.[
Reported treatments for regurgitation due to high-flow upper-limb AVF include termination of the AVF (ligation or occlusion), angioplasty with or without stenting of stenoocclusive central veins, and anticoagulant therapy; the last two preserve the AVF.[
Our patient reportedly had IJV regurgitation due to an extensive high brachio-brachial AVF flow, without CVOD. The IJV valve was visible to the patient. IJV regurgitation observed in the supine position increased under the Valsalva maneuver, suggesting IJV valve incompetence. The flow of the left brachial artery was excessive (up to 3150 ml/min). In contrast to previously reported cases, the IJV regurgitation in our patient was thought to be caused by IJV valve incompetence and excessive AVF flow, not by CVOD. The findings suggested that compressing the brachial artery to achieve optimal blood flow did not prevent regurgitation. DSA revealed that all cerebral venous outlets utilized the right IJV. Ligation of the left IJV was the treatment implemented to achieve preservation of functional AVF and termination of IJV regurgitation. This treatment may have been the best course of action for this patient as it urgently reduced intracranial pressure, resulting in good neurological outcomes.
This study had limitations as it was a case report that may have incidentally shown a positive clinical outcome. The patient had not been observed for a long period in terms of general condition, including cardiac outcome and AVF patency, as well as neurological condition.
CONCLUSION
We report a case of symptomatic cerebral venous reflux due to IJV regurgitation without CVOD. IJV regurgitation without CVOD is rare. The short-term outcome after IJV ligation may be positive in the patient who was confirmed to have a normal cerebral venous return route independent of the refluxed IJV after thorough observation. Long-term follow-up and further accumulation of cases are crucial to confirm the relevance of these treatment results.
DECLARATIONS
Ethics approval and consent to participate
Not applicable.
Availability of data and materials
The data that support the findings of this case are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Caiza-Zambrano F, Palacio CM, Garbugino S, Gonzalez FM, Biolcati MB, Saucedo MÁ. Central venous reflux, a rare cause of neurological manifestations in hemodialysis patients: A case report and literature review. Neurointervention. 2022. 17: 58-64
2. Chang S, Masaryk TJ, Lee MS. Optic nerve edema: complication of peripheral hemodialysis shunt. Semin Ophthalmol. 2004. 19: 88-90
3. Chuang YM, Hu HH. Cough headache and thoracic inlet valvular competence in uremia. Eur Neurol. 2005. 53: 78-80
4. Cuadra SA, Padberg FT, Turbin RE, Farkas J, Frohman LP. Cerebral venous hypertension and blindness: A reversible complication. J Vasc Surg. 2005. 42: 792-5
5. Dresser LP, McKinney WM. Anatomic and pathophysiologic studies of the human internal jugular valve. Am J Surg. 1987. 154: 220-4
6. Fisher J, Vaghaiwalla F, Tsitlik J, Levin H, Brinker J, Weisfeldt M. Determinants and clinical significance of jugular venous valve competence. Circulation. 1982. 65: 188-96
7. Harmon JV, Edwards WD. Venous valves in subclavian and internal jugular veins. Frequency, position, and structure in 100 autopsy cases. Am J Cardiovasc Pathol. 1987. 1: 51-4
8. Hartmann A, Mast H, Stapf C, Koch HC, Marx P. Peripheral hemodialysis shunt with intracranial venous congestion. Stroke. 2001. 32: 2945-6
9. Haruma J, Escalard S, Smajda S, Piotin M. Left temporal hemorrhage caused by cerebral venous reflux of a brachiobrachial hemodialysis fistula. Neuroradiology. 2020. 62: 1341-4
10. Hashimoto T, Akagi D, Yamamoto S, Suhara M, Sato O, Deguchi J. Short interposition with a small-diameter prosthetic graft for flow reduction of a high-flow arteriovenous fistula. J Vasc Surg. 2021. 73: 285-90
11. Herzig DW, Stemer AB, Bell RS, Liu AH, Armonda RA, Bank WO. Neurological sequelae from brachiocephalic vein stenosis. J Neurosurg. 2013. 118: 1058-62
12. Iguchi T, Harada M, Kurihara S, Ichikawa T, Satoh S, Kobayashi M. Neurological symptoms due to intracranial venous congestion in a hemodialysis patient with arteriovenous shunted flow. Kidney Int Rep. 2020. 5: 2097-101
13. Lal SM, Twardowski ZJ, Van Stone J, Keniston D, Scott WJ, Berg GG. Benign intracranial hypertension: A complication of subclavian vein catheterization and arteriovenous fistula. Am J Kidney Dis. 1986. 8: 262-4
14. Mackay DD, Biousse V. Hemodialysis graft-induced intracranial hypertension. Neurol Clin Pract. 2015. 5: 494-7
15. MacRae JM, Levin A, Belenkie I. The cardiovascular effects of arteriovenous fistulas in chronic kidney disease: A cause for concern?. Semin Dial. 2006. 19: 349-52
16. Mirza MH, Schwertner A, Kohlbrenner R, Dowd CF, Narsinh KH. Intracranial hemorrhage due to central venous occlusion from hemodialysis access: A case report. Interdiscip Neurosurg. 2021. 24: 101081
17. Molina JC, Martinez-Vea A, Riu S, Callizo J, Barbod A, Garcia C. Pseudotumor cerebri: An unusual complication of brachiocephalic vein thrombosis associated with hemodialysis catheters. Am J Kidney Dis. 1998. 31: E3
18. Nishijima H, Tomiyama M, Haga R, Ueno T, Miki Y, Arai A. Venous cerebral infarction in a patient with peripheral hemodialysis shunt and occlusion of the left brachiocephalic vein. J Stroke Cerebrovasc Dis. 2011. 20: 381-3
19. Nishimoto H, Ogasawara K, Miura K, Ohmama S, Kashimura H, Ogawa A. Acute intracranial hypertension due to occlusion of the brachiocephalic vein in a patient undergoing hemodialysis. Cerebrovasc Dis. 2005. 20: 207-8
20. Prasad V, Baghai S, Gandhi D, Moeslein F, Jindal G. Cerebral infarction due to central vein occlusion in a hemodialysis patient. J Neuroimaging. 2015. 25: 494-6
21. Roh GU, Kim WO, Rha KH, Lee BH, Jeong HW, Na S. Prevalence and impact of incompetence of internal jugular valve on postoperative cognitive dysfunction in elderly patients undergoing robot-assisted laparoscopic radical prostatectomy. Arch Gerontol Geriatr. 2016. 64: 167-71
22. Saha MK, Hamieh T, Larkin B, Mcmillan W. Cerebral hemorrhage due to internal jugular vein stenosis in a hemodialysis patient. Clin Exp Nephrol. 2012. 16: 345-9
23. Salama GR, Farinhas JM, Pasquale DD, Wertenbaker C, Bello JA. Central venous occlusion mimics carotid cavernous fistula: A case report and review of the literature. Clin Imaging. 2014. 38: 884-7
24. Samaniego EA, Abrams KJ, Dabus G, Starr R, Linfante I. Severe venous congestive encephalopathy secondary to a dialysis arteriovenous graft. J Neurointerv Surg. 2013. 5: e37
25. Simon MA, Duffis EJ, Curi MA, Turbin RE, Prestigiacomo CJ, Frohman LP. Papilledema due to a permanent catheter for renal dialysis and an arteriovenous fistula: A “two hit” hypothesis. J Neuroophthalmol. 2014. 34: 29-33
26. Varelas PN, Bertorini TE, Halford H. Bilateral ophthalmoplegia and exophthalmos complicating central hemodialysis catheter placement. Am J Kidney Dis. 1999. 33: 966-9
27. Watson RR, Russo C. Upper extremity arteriovenous dialysis fistula resulting in cavernous sinus arterialized blood flow. AJNR Am J Neuroradiol. 2007. 28: 1155-6