- Department of Neurosurgery, Highly Specialized Hospital of National Importance “Garibaldi,” Catania, Italy
- Department of Neurosurgery, Cannizzaro Hospital, Catania, Italy.
Correspondence Address:
Gianluca Scalia
Department of Neurosurgery, Highly Specialized Hospital of National Importance “Garibaldi,” Catania, Italy
DOI:10.25259/SNI_259_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Giancarlo Ponzo, Giuseppe Emmanuele Umana, Massimiliano Giuffrida, Massimo Furnari, Giovanni Federico Nicoletti, Gianluca Scalia. Intramedullary craniovertebral junction metastasis leading to the diagnosis of underlying renal cell carcinoma. 13-Jun-2020;11:152
How to cite this URL: Giancarlo Ponzo, Giuseppe Emmanuele Umana, Massimiliano Giuffrida, Massimo Furnari, Giovanni Federico Nicoletti, Gianluca Scalia. Intramedullary craniovertebral junction metastasis leading to the diagnosis of underlying renal cell carcinoma. 13-Jun-2020;11:152. Available from: https://surgicalneurologyint.com/surgicalint-articles/10081/
Abstract
Background: Intramedullary spinal cord metastases represent 4–8.5% of the central nervous system metastases and affect only 0.1–0.4% of all patients. Those originating from renal cell carcinoma (RCC) are extremely rare. Of the eight patients described in the literature with metastatic RCC and intramedullary cord lesion, only five were found in the cervical spine. Here, the authors add a 6th case involving an RCC intramedullary metastasis at the C1–C2 level.
Case Description: A 78-year-old male patient presented with intermittent cervicalgia of 5 months duration accompanied by few weeks of a progressive severe right hemiparesis, up to hemiplegia. The magnetic resonance imaging (MRI) examination revealed an intramedullary expansive lesion measuring 10 mm×15 mm at the C1–C2 level; it readily enhanced with contrast. A total body computed tomography (CT) scan documented an 85 mm mass involving the right kidney, extending to the ipsilateral adrenal gland, and posteriorly infiltrating the ipsilateral psoas muscle. The subsequent CT-guided fine-needle biopsy confirmed the diagnosis of an RCC (Stage IV). The patient next underwent total surgical total removal of the C1–C2 intramedullary mass, following which he exhibited a slight motor improvement, with the right hemiparesis (2/5). He died after 14 months due to global RCC tumor progression.
Conclusion: The present case highlights that a patient without a prior known diagnosis of RCC may present with an intramedullary C1–C2 metastasis. In such cases, global staging is critical to determine whether primary lesion resection versus excision of metastases (e.g., in this case, the C1–C2 intramedullary tumor) are warranted.
Keywords: Craniovertebral junction, Intramedullary, Metastasis, Myelotomy, Renal cell carcinoma
INTRODUCTION
Intramedullary spinal cord metastases (IMSCMs) represent the 4–8.5% of the central nervous system metastases, affecting 0.1–0.4% of all patients.[
CASE REPORT
Medical history and physical examination
A 78-year-old male patient presented with 5 months of intermittent cervicalgia and several weeks of a progressive right hemiparesis, up to hemiplegia (0/5), brisk upper and lower extremity reflexes, bilateral Hoffmann’s and Babinski signs, left hemisensory dysesthesias, and urinary incontinence.
Diagnostic imaging
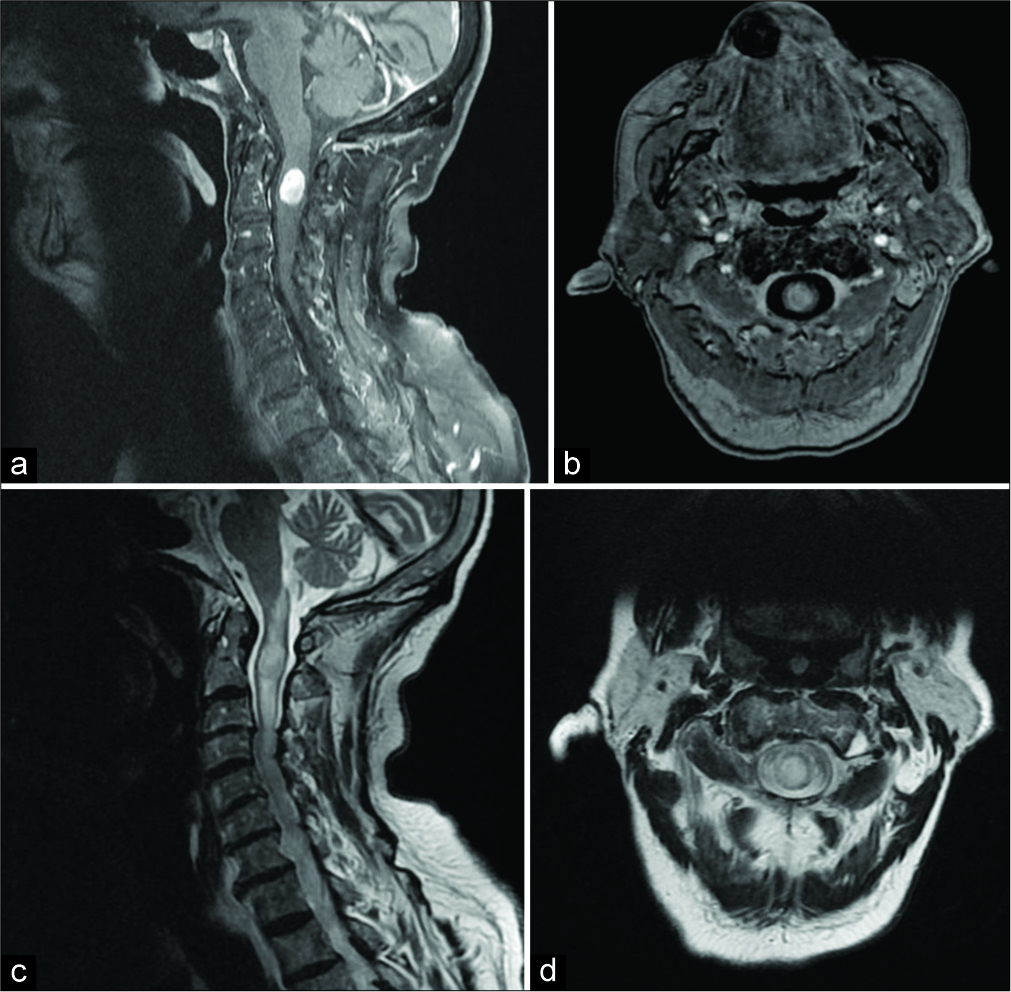
The cervical spinal MRI revealed an intramedullary expansive lesion (10 mm×15 mm) at C1–C2 that markedly enhanced with gadolinium [
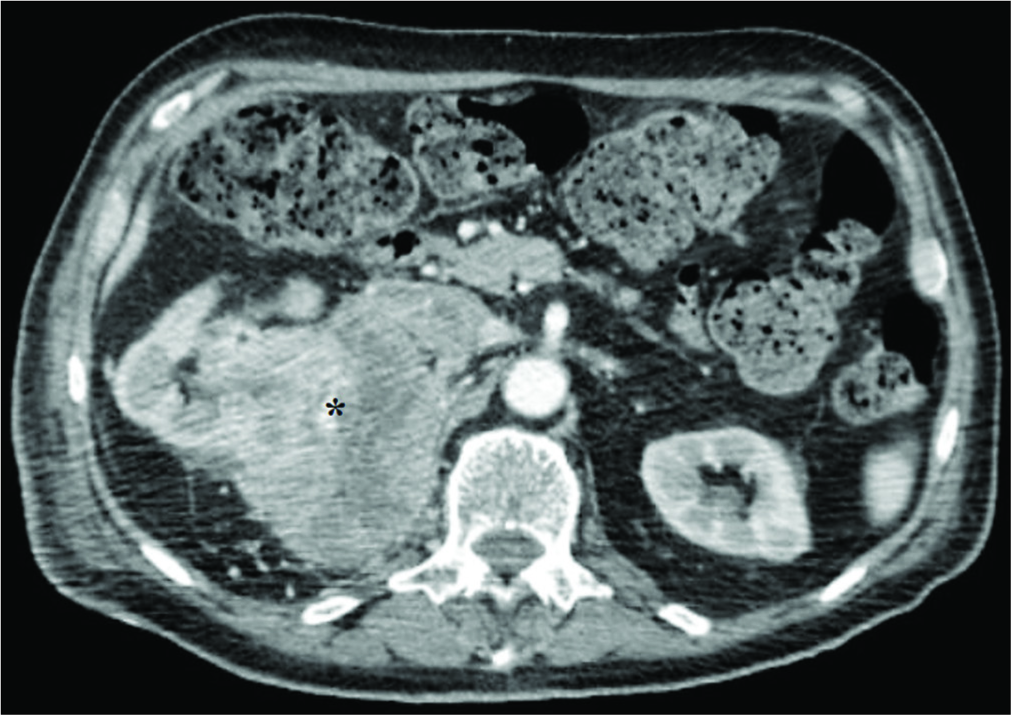
Figure 2:
Axial abdominal contrast-enhanced computed tomography scan image showing a voluminous mass (about 85 mm) (black asterisk) involving the upper polar region and the middle third of the right kidney, the ipsilateral adrenal gland, and extends posteriorly to infiltrate the ipsilateral psoas muscle. This lesion, which presents an inhomogeneous hypodense aspect with hypervascular foci in this context, is associated with collateral circles in the peri- and pararenal space, with the infiltration of the upper right calyxes. A neoplastic thrombosis of the renal vein and inferior vena cava in the subhepatic tract is also present and may explain hematogenous spread through Batson’s venous plexus.
Surgical treatment
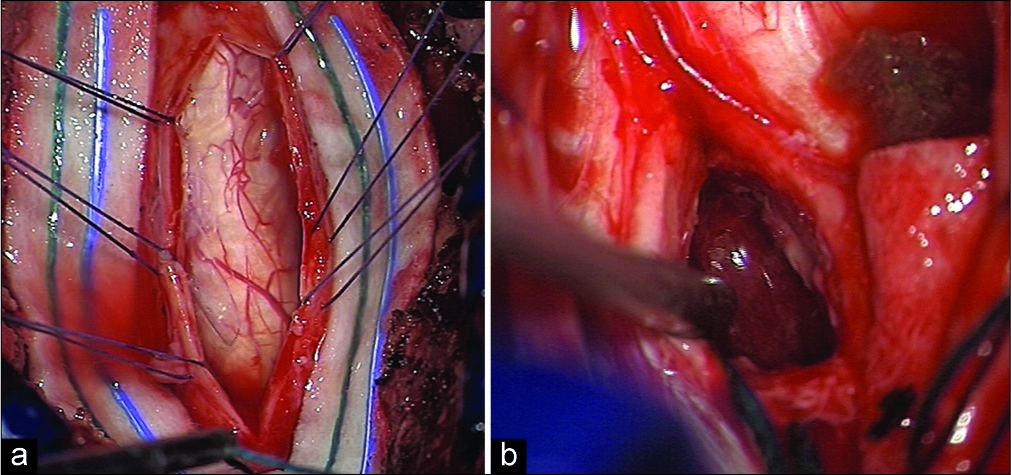
Utilizing intraoperative neurophysiological monitoring, a C1–C2 laminectomy was performed. Through a posterior C2, myelotomy, and the lesion were macroscopically fully resected [
Figure 3:
Intraoperative findings during microsurgical removal of the lesion: a good exposure of the posterior surface of the spinal cord at level C1–C2 after opening the dura mater is performed (a). After arachnoid dissection and preservation of the posterior spinal arteries, the posterior median sulcus is identified and the posterior myelotomy is performed, with access to the intramedullary lesion which shows a reddish-gray and highly vascularized appearance (b).
Histology
The histological examination revealed large cells with marked anaplasia. Immunostaining was negative for cytokeratin, GFAP, S-100, and HMB-45 but positive for intermediate vimentin filaments. Together, these studies confirmed the diagnosis of an RCC.
Postoperative course and follow-up
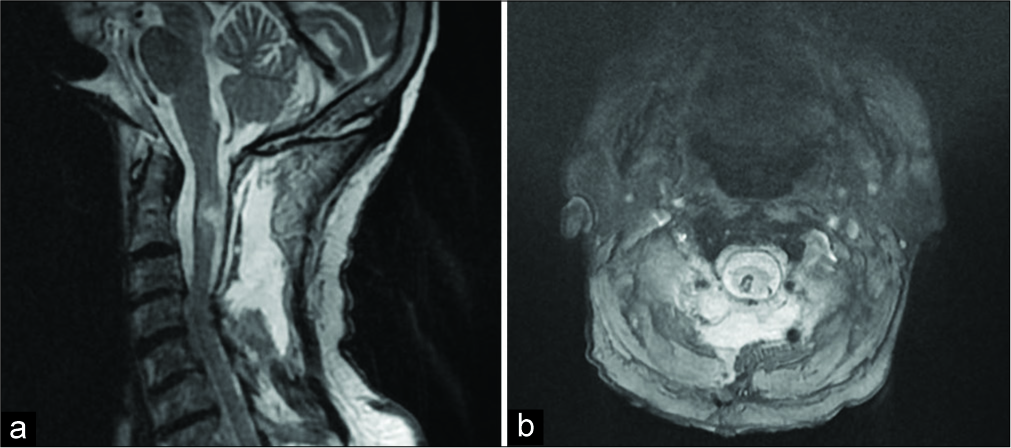
The 1-week postoperative cervical spine MRI showed postoperative changes, but full lesion excision [
DISCUSSION
Patients affected by RCC that develop IMSCMs are usually male (83%). These IMSCMs occur in the cervical spine in 47% of cases. Patients typically present with limb weakness (72%), dysesthesias, and urinary incontinence (50%).[
CONCLUSION
Patients may present with symptoms/signs of and intramedullary C1–C2 spinal cord metastasis as the first sign of RCC. As performed in this case, global staging should document the origin and extent of the primary lesion and other systemic metastases and help determine whether or not excision of a C1–C2 IMSCM is warranted.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Asadi M, Rokni-Yazdi H, Salehinia F, Allameh FS. Metastatic renal 1 cell carcinoma initially presented with an intramedullary spinal cord lesion: A case report. Cases J. 2009. 2: 7805-
2. Barrie U, Elguindy M, Pernik M, Adeyemo E, Aoun SG, Hall K. Intramedullary spinal metastatic renal cell carcinoma: Systematic review of disease presentation, treatment, and prognosis with case illustration. World Neurosurg. 2020. 134: 584-93
3. De Meerleer G, Khoo V, Escudier B, Joniau S, Bossi A, Ost P. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 2014. 15: e170-7
4. Donovan DJ, Freeman JH. Solitary intramedullary spinal cord tumor presenting as the initial manifestation of metastatic renal cell carcinoma: Case report. Spine (Phila Pa 1976). 2006. 31: E460-3
5. Fakih M, Schiff D, Erlich R, Logan TF. Intramedullary spinal cord metastasis (ISCM) in renal cell carcinoma: A series of six cases. Ann Oncol. 2001. 12: 1173-7
6. Gaylor JB, Howie JW. Brown-sequard syndrome: A case of unusual aetiology. J Neurol Psychiatry. 1938. 1: 301-5
7. G5.301de la Riva AG, Isla A, Perez-Lopez C, Budke M, Gutierrez M, Frutos R. Intramedullary spinal cord metastasis as the first manifestation of a renal carcinoma. Neurocirugia (Astur). 2005. 16: 359-64
8. Hrabalek L. Intramedullary spinal cord metastases: Review of the literature. Biomed Pa Med Fac Univ Palacky Olomouc Czech Repub. 2010. 154: 117-22
9. Kalayci M, Cagavi F, Gul S, Yenidunya S, Acikgoz B. Intramedullary spinal cord31 metastases: Diagnosis and treatment an illustrated review. Acta Neurochir (Wien). 2004. 146: 1347-54
10. Kawakami Y, Mair WG. Haematomyelia associated with anticoagulant therapy, an intramedullary ependymoma and Schwann cells. Acta Neuropathol. 1973. 26: 253-8
11. Park J, Chung SW, Kim KT, Cho DC, Hwang JH, Sung JK. Intramedullary spinal cord metastasis in renal cell 37 carcinoma: A case report of the surgical experience. J Korean Med Sci. 2013. 28: 1253-6
12. Payer S, Mende KC, Westphal M, Eicker SO. Intramedullary spinal cord metastases: An increasingly common diagnosis. Neurosurg Focus. 2015. 39: E15-
13. Saeed H, Patel R, Thakkar J, Hamoodi L, Chen L, Villano JL. Multimodality therapy17 improves survival in intramedullary spinal cord metastasis of lung primary. Hematol Oncol Stem18 Cell Ther. 2017. 10: 143-50
14. Schijns OE, Kurt E, Wessels P, Luijckx GJ, Beuls EA. Intramedullary spinal cord metastasis as a first manifestation of a renal cell carcinoma: Report of a case and review of the literature. Clin Neurol Neurosurg. 2000. 102: 249-54
15. Tsai TH, Lin IC, Lin PC, Wu CH, Lin CL, Su YF. Intramedullary spinal cord metastasis 26 from colon cancer: Analysis of 19 reported cases. Spinal Cord Ser Cases. 2016. 2: 15026-