- Department of Neurosurgery, University of Passo Fundo, Passo Fundo, Rio Grande do Sul, Brazil
- Department of Neurosurgery, São Vicente de Paulo Hospital, Passo Fundo, Rio Grande do Sul, Brazil.
Correspondence Address:
Eduardo Cattapan Piovesan, Department of Neurosurgery, University of Passo Fundo, Passo Fundo, Rio Grande do Sul, Brazil.
DOI:10.25259/SNI_252_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Eduardo Cattapan Piovesan1, Werner Petry Silva2, Adroaldo Baseggio Mallmann2, Felipe Severo Lanzini1, Bruna Zanatta de Freitas1, Francisco Costa Beber Lemanski1, Charles André Carazzo1,2. Intramedullary hemangioblastoma of the thoracic cord with a microsurgical approach: A case report and literature review. 14-Apr-2023;14:137
How to cite this URL: Eduardo Cattapan Piovesan1, Werner Petry Silva2, Adroaldo Baseggio Mallmann2, Felipe Severo Lanzini1, Bruna Zanatta de Freitas1, Francisco Costa Beber Lemanski1, Charles André Carazzo1,2. Intramedullary hemangioblastoma of the thoracic cord with a microsurgical approach: A case report and literature review. 14-Apr-2023;14:137. Available from: https://surgicalneurologyint.com/surgicalint-articles/intramedullary-hemangioblastoma-of-the-thoracic-cord-with-a-microsurgical-approach-a-case-report-and-literature-review/
Abstract
Background: Spinal cord hemangioblastomas (HBs) account for 2–15% of all spinal cord neoplasms. They are the third most common primary intramedullary tumor (1–5%). Here, 72-year-old female presented with a thoracic intramedullary spinal HB that responded well to surgery.
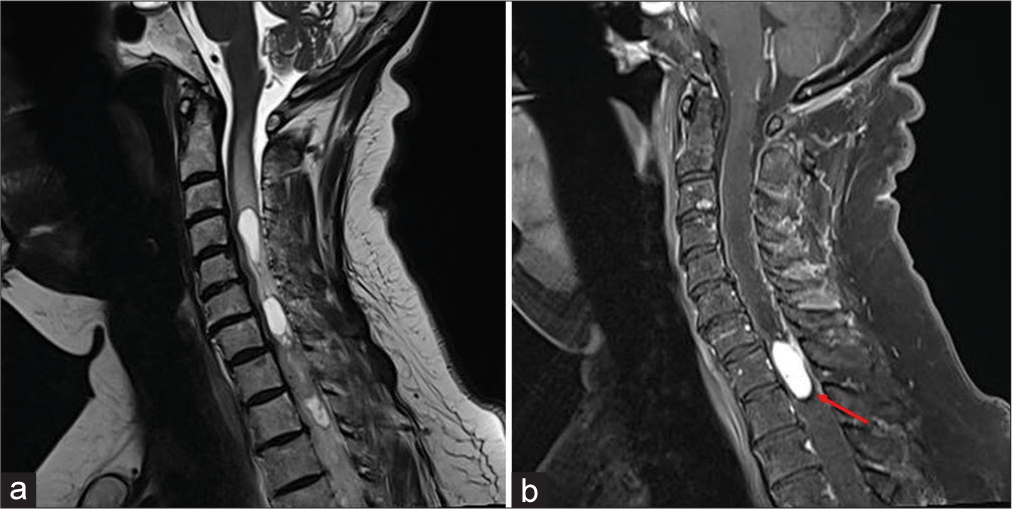
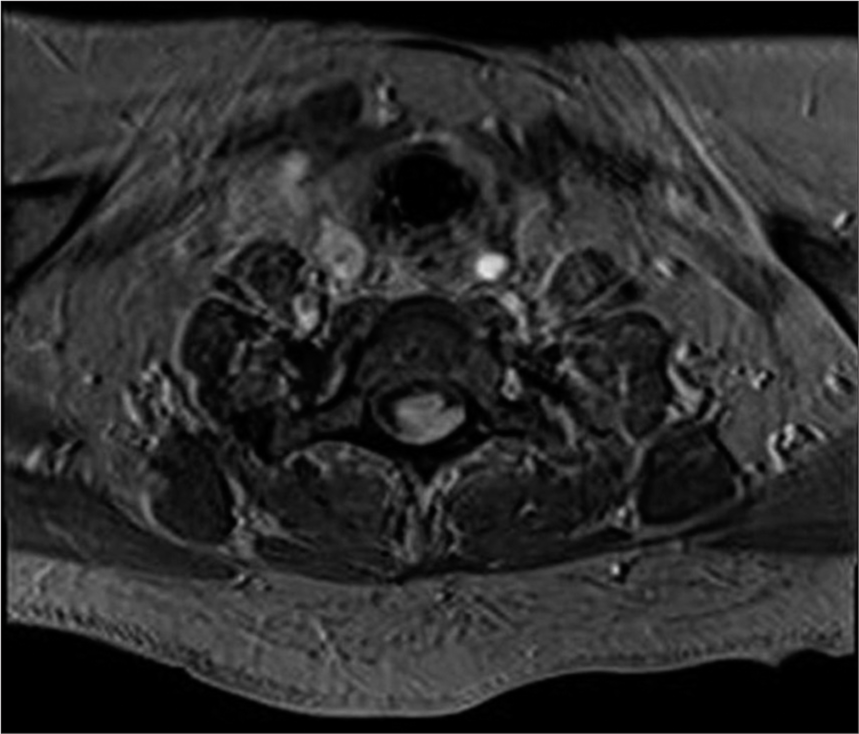
Case Description: A 72-year-old female presented with a 3–4 years of progressive paresthesias and paraparesis. On examination, she exhibited diffuse distal weakness of the lower extremities. The magnetic resonance scan showed an intramedullary expansive lesion at the T1–T2 level that markedly enhanced with contrast with both proximal and distal hydromyelia. Surgery included a C7 partial and T1–T2 total laminectomies performed under microscope visualization with intraoperative monitoring. At surgery, there was a well-documented cleavage plane between the tumor and the cord; excision was facilitated using the cavitron ultrasonic surgical aspirator device.
Conclusion: Surgery is the gold standard treatment for treating/resecting HBs and should include utilization of an operating microscope and intraoperative monitoring.
Keywords: Hemangioblastoma, Neurosurgery, Spine surgery, Spine, Spine tumor
INTRODUCTION
Spinal hemangioblastomas (HBs) are benign and highly vascular tumors that can occur anywhere throughout the central nervous system. They account for 2–15% of all spinal cord neoplasm and are the third most common primary intramedullary tumors (1–5%). Notably, they most commonly involve the cervical spine and are often associated with perilesional edema, and/or peritumoral cysts.[
CASE REPORT
Clinical presentation
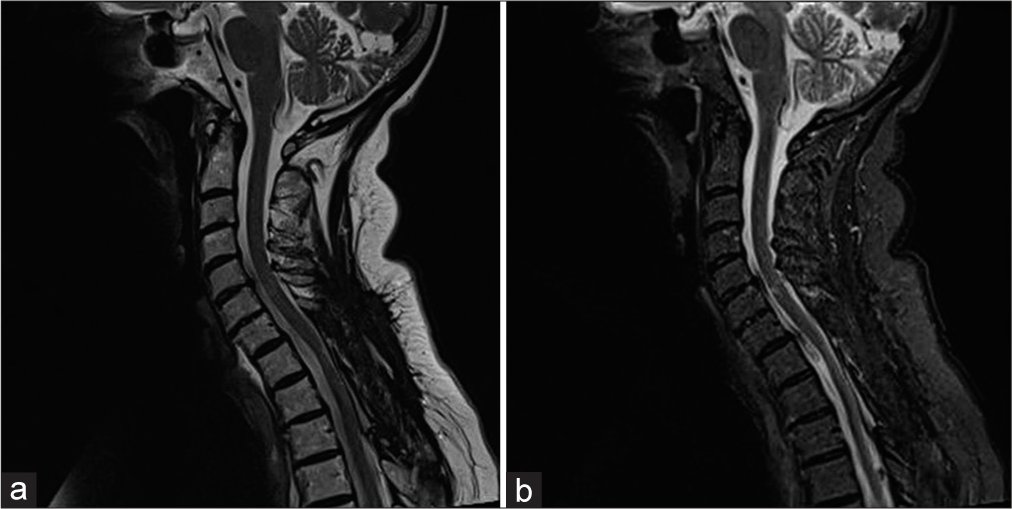
A 72-year-old female presented with a 6 month history of progressive paresthesias and paraparesis. On examination, she exhibited distal lower extremity weakness (4/5 level dorsiflexion along with diffuse lower extremity hyperreflexia and bilateral Babinski signs). The magnetic resonance imaging (MRI) demonstrated an intramedullary expansive lesion at the T1–T2 level that markedly enhanced with contrast; it was also accompanied by cephalad and caudad hydromyelia [
Surgery
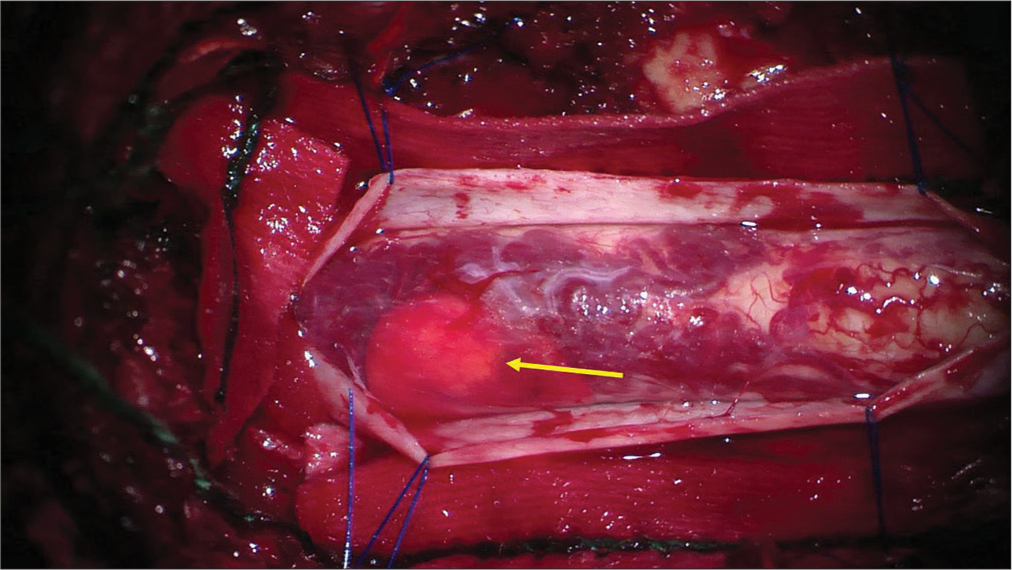
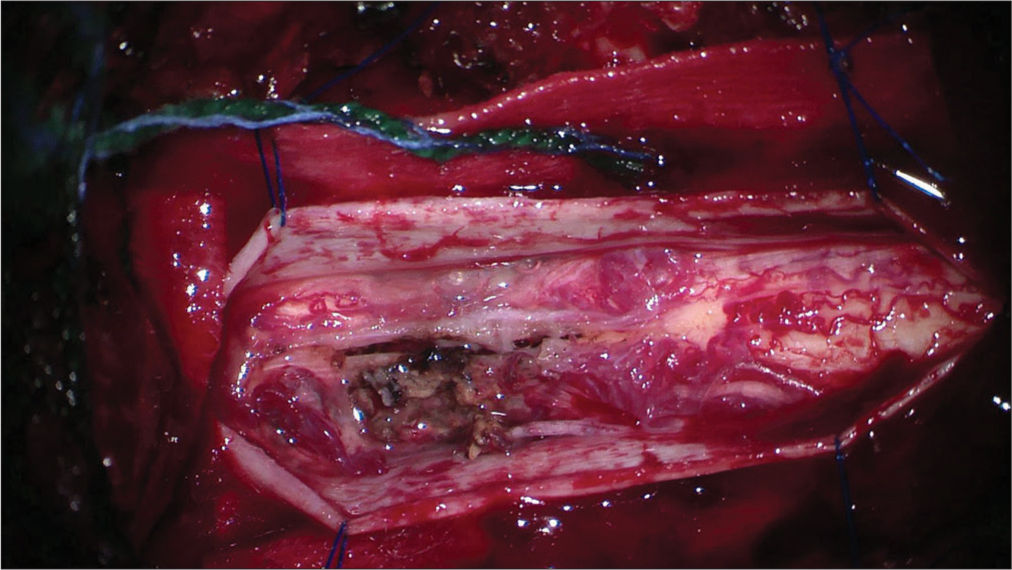
The patient underwent for a C7 partial and T1–T2 total laminectomies. Using microscope visualization and intraoperative monitoring, the dura was opened revealing an expansive and very vascular intramedullary cord lesion [
Pathology
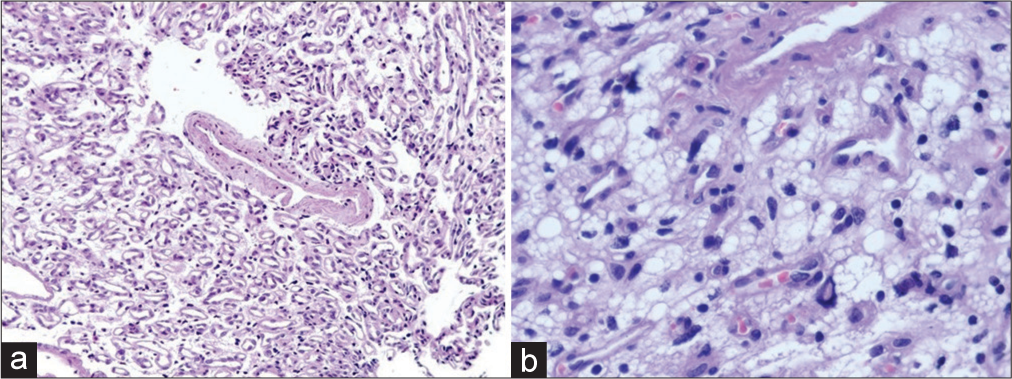
The histopathological examination confirmed the diagnosis of an HB. The tumor included a rich vascular network and vacuolated cells with mild nuclear enlargement and clear cytoplasm. Immunohistochemical staining revealed stromal cells positive for inhibin A, S100, and CD31, and was immunonegative for CD56, and AE1-3 [
Postoperatively
Postoperatively, the patient’s paraparesis markedly improved. The postoperative MRI revealed that the tumor had been totally removed, and the syrinxes/hydromyelia cephalad/ caudad were diminished. One-year later, the patient was fully neurologically intact, and the magnetic resonance (MR) continued to confirm the lack of tumor recurrence along with further reduction of the cephalad/caudad syrinxes/ hydromyelia [
DISCUSSION
Patients typically present with symptoms/signs of spinal HB during the fourth decade of life.[
MRI gold standard examination for HB
MRI remains the gold standard for diagnosing HB; lesions are typically iso- to hypointense on T1-weighted sequences, and iso- to hyperintense on T2-weighted studies; further, they markedly enhance with contrast.[
Indications for surgery
For patients with HB, the clinical and radiographic findings both determine whether surgical intervention is warranted. The optimal surgical management includes gross-total resection that is typically achieved in over 90% of the cases; this usually results in a 96% incidence of full functional recovery of the cases. Postoperatively, patients’ symptoms often regress within 1–2 postoperative weeks, while follow-up contrast MR studies help confirm complete tumor resection, along with documenting regression of cephalad/caudad cysts (i.e., syrinxes/hydromyelia over 3–6 months).[
CONCLUSION
A 72-year-old female presented with myelopathy/paraparesis attributed to a T1–T2 HB. Following complete tumor resection, the patient regained normal neurological function, and the follow-up MR up to 6 months lateral confirmed no tumor recurrence and regression of the cephalad/caudad syrinxes/hydromyelia.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Baker KB, Moran CJ, Wippold FJ, Smirniotopoulos JG, Rodriguez FJ, Meyers SP. MR imaging of spinal hemangioblastoma. AJR Am J Roentgenol. 2012. 174: 377-82
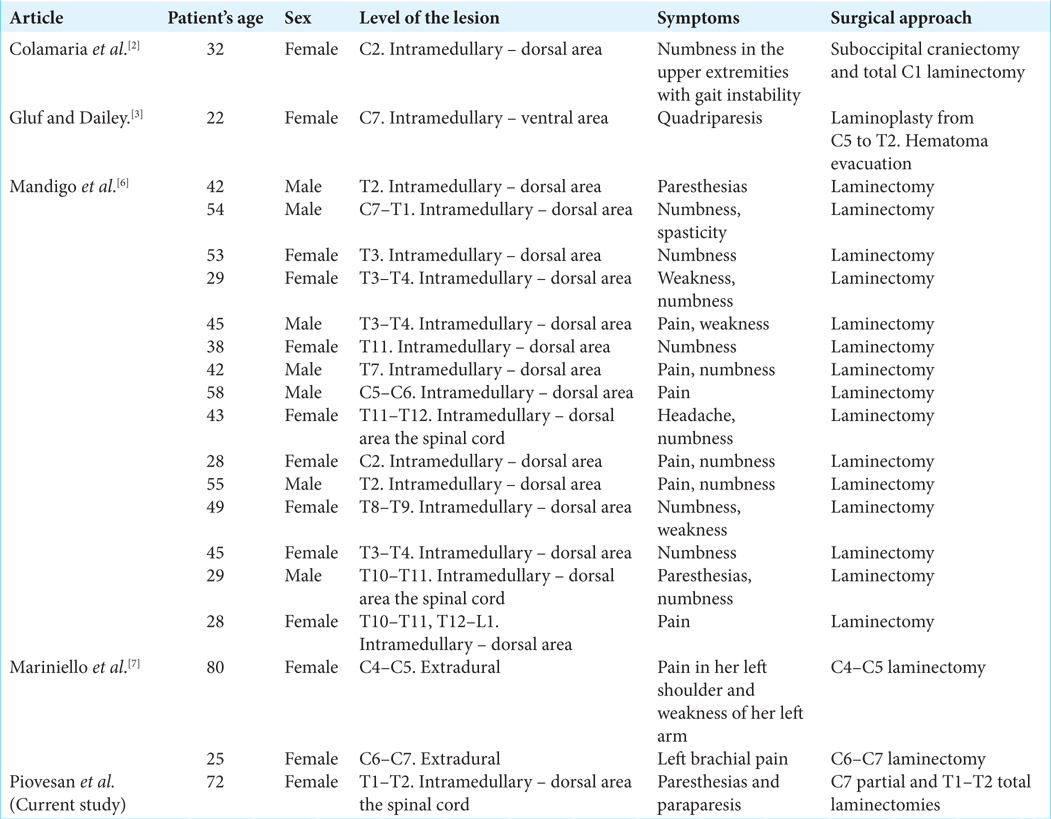
2. Colamaria A, Fochi NP, Laguado YA, Blagia M, Leone A, Carbone F. Cervical intra and extramedullary hemangioblastoma with associated syringomyelia: A case report and review of the literature. Surg Neurol Int. 2022. 13: 448
3. Gluf WM, Dailey AT. Hemorrhagic intramedullary hemangioblastoma of the cervical spinal cord presenting with acute-onset quadriparesis: Case report and review of the literature. J Spinal Cord Med. 2014. 37: 791-4
4. Intramedullary Spinal Cord Tumors-StatPearls-NCBI Bookshelf. Available from: https://www.ncbi.nlm.nih.gov/books/NBK442031 [Last accessed on 2023 Jan 06].
5. Lonser RR, Oldfield EH. Spinal cord hemangioblastomas. Neurosurg Clin N Am. 2006. 17: 37-44
6. Mandigo CE, Ogden AT, Angevine PD, McCormick PC. Operative management of spinal hemangioblastoma. Neurosurgery. 2009. 65: 1166-77
7. Mariniello G, Corvino S, Corazzelli G, Franca RA, de Caro MD, Maiuri F. Spinal cervical extradural hemangioblastoma. J Craniovertebr Junction Spine. 2022. 13: 192-7
8. Messerer M, Cossu G, Pralong E, Daniel RT. Intramedullary hemangioblastoma: Microsurgical resection technique. Neurochirurgie. 2017. 63: 376-80
9. Yasuda T, Hasegawa T, Yamato Y, Kobayashi S, Togawa D, Banno T. Relationship between spinal hemangioblastoma location and age. Asian Spine J. 2016. 10: 309-13
10. Yoda RA, Cimino PJ. Neuropathologic features of central nervous system hemangioblastoma. J Pathol Transl Med. 2022. 56: 115-25