- Neurosurgery Resident, Regional Hospital of Presidente Prudente, São Paulo, Brazil,
- Pediatrics Infectious Disease, Regional Hospital of Presidente Prudente, São Paulo, Brazil,
- Postdoctoral Fellowship, Lyerly Neurosurgery, Baptist Neurological Institute, Jacksonville, Florida, United States,
- Neurosurgeon, Regional Hospital of Presidente Prudente, São Paulo, Brazil.
Correspondence Address:
José Renan Miranda Cavalcante Filho, Neurosurgery Resident, Regional Hospital of Presidente Prudente, São Paulo, Brazil.
DOI:10.25259/SNI_1064_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: José Renan Miranda Cavalcante Filho1, Patrícia Rodrigues Naufal Spir2, Gustavo Maldonado Cortez3, Adib Saraty Malveira1, Felipe Franco Pinheiro Gaia4. Intramedullary histoplasmosis lesion in children: A case report. 11-Mar-2022;13:83
How to cite this URL: José Renan Miranda Cavalcante Filho1, Patrícia Rodrigues Naufal Spir2, Gustavo Maldonado Cortez3, Adib Saraty Malveira1, Felipe Franco Pinheiro Gaia4. Intramedullary histoplasmosis lesion in children: A case report. 11-Mar-2022;13:83. Available from: https://surgicalneurologyint.com/surgicalint-articles/11439/
Abstract
Background: Histoplasmosis is a fungal disease endemic in some regions of the United States of America, Canada, and Latin America. The geographic characteristics, humidity, soil, and climate are responsible for such distribution. In Brazil, there are case reports of histoplasmosis throughout its territory, being considered an endemic region. It is considered an opportunistic disease, affecting mostly immunocompromised patients. To the present date, scientific publications dealing with pediatric cases of histoplasmosis are restricted to case series. Spinal cord injuries caused by histoplasmosis are rare, even in the adult population, being described in few studies.
Case Description: The present report deals with a 4-year-old patient, from the southeast region of Brazil, who started a condition of fever, weight loss, cervicobrachialgia, and symmetrical tetraparesis, with evolution over 2 months. In the diagnostic investigation, she was found to have primary immunodeficiency and neuroimaging examinations showed a cervical spinal cord lesion at the level of C4-C6. The anatomopathological diagnosis of histoplasmosis was possible after surgery for decompression and biopsy of the lesion.
Conclusion: According to our research, there are no reports in the literature that address the situation of spinal cord compression syndrome due to histoplasmosis in the pediatric population.
Keywords: Histoplasmosis, Neuroinfection, Neurosurgery, Pediatrics, Spinal cord lesion
INTRODUCTION
Histoplasmosis is a fungal disease endemic in some regions of the United States of America, Canada, and Latin America. The geographic characteristics, humidity, soil, and climate are responsible for such distribution.[
Dissemination occurs mainly through hematogenous or contiguity. The most common neurological symptoms are chronic meningitis, hydrocephalus, and encephalitis, in addition to focal lesions in the brain and spinal cord.[
Spinal cord injuries caused by histoplasmosis are rare, even in the adult population, being described in few studies.[
The present report deals with a 4-year-old patient, from the southeast region of Brazil, she was found to have primary immunodeficiency and neuroimaging examinations showed a cervical spinal cord lesion at the level of C4-C6. The anatomopathological diagnosis of histoplasmosis was possible after surgery for decompression and biopsy of the lesion. So far, according to our research, there are no reports in the literature that address the situation of spinal cord compression syndrome due to histoplasmosis in the pediatric population.
CASE DESCRIPTION
A 4-year-old girl with no previous comorbidities is admitted to the emergency room for 2 months, presenting with asthenia, weight loss, neck pain, difficulty in walking, and frequent falls for 2 months, evolving for 1 week with fever and progressive tetraparesis in all four limbs. On neurological examination, the patient had Glasgow 15, isochoric and photoreactive pupils, dysbasia, axial and appendicular ataxia, as well as alterations of the upper motor neuron syndrome (spastic tetraparesis, bilateral Hoffmann and Babinski signs, and global hyperreflexia).
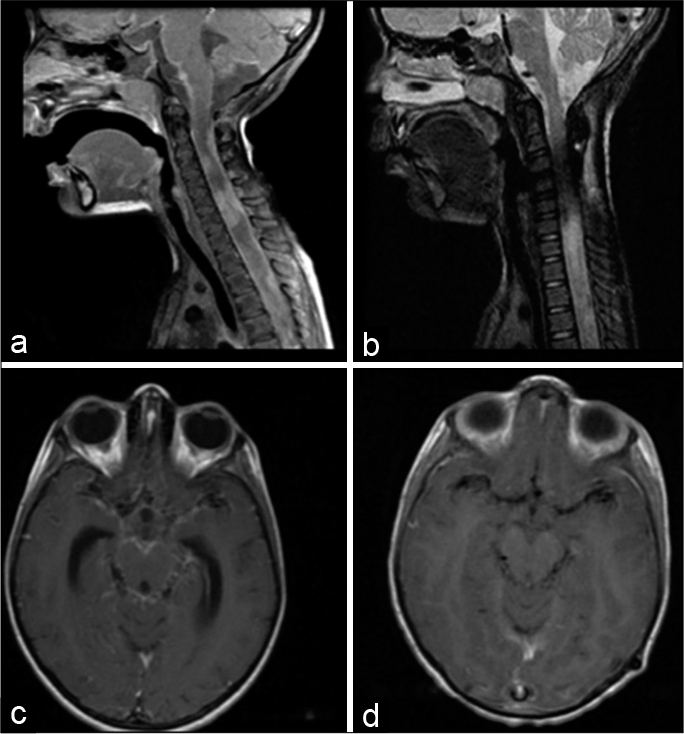
In the diagnostic investigation, neuroaxis magnetic resonance imaging (MRI) was performed, which showed multiple intramedullary expansile formations with diffuse leptomeningeal enhancement of the structures and spinal cord, as well as intraparenchymal enhancement in the brainstem, suggesting areas of cerebritis [
Figure 1:
(a) Pretreatment T1-weighted magnetic resonance imaging (MRI) of cervical spine, sagittal view, showing multiple contrast-enhancing solid intramedullary expansive formations. The intramedullary lesion between C4 and C6 stands out, with dimensions: 14 mm in diameter caudal, 7 mm anteroposterior, and 8.2 mm laterolateral, as well as leptomeningeal enhancement. (b) T2-weighted posttreatment MRI with significant reduction in meningeal enhancement, denoting the resolution of the infectious process after the implemented therapy; (c) pretreatment T1-weighted axial MRI of the brain showing the important diffuse leptomeningeal enhancement in the basal region of the brain, typical of granulomatous diseases. (d) MRI after 42 days of T1-weighted treatment showing the important reduction in meningeal enhancement.
On the 11th day of hospitalization, the patient underwent microsurgery for the resection of an expansive intramedullary lesion by C4-C6 posterior laminectomy, a procedure that occurred uneventfully. However, on the 6th postoperative day, the child evolved with a worsening level of consciousness, vomiting, and prostration. An urgent cranial computed tomography was performed, which showed acute hydrocephalus, and an external ventricular drainage (EVD) was then indicated for resolution of the condition. After the EVD procedure, the patient showed a significant improvement of the sensorium and the intraoperative cerebrospinal fluid (CSF) analysis showed erythrocytes 220, leukocytes 12.5 (72% lymphocytes; monocytes 3%; and neutrophils 25%), glucose 58, and proteins 29.
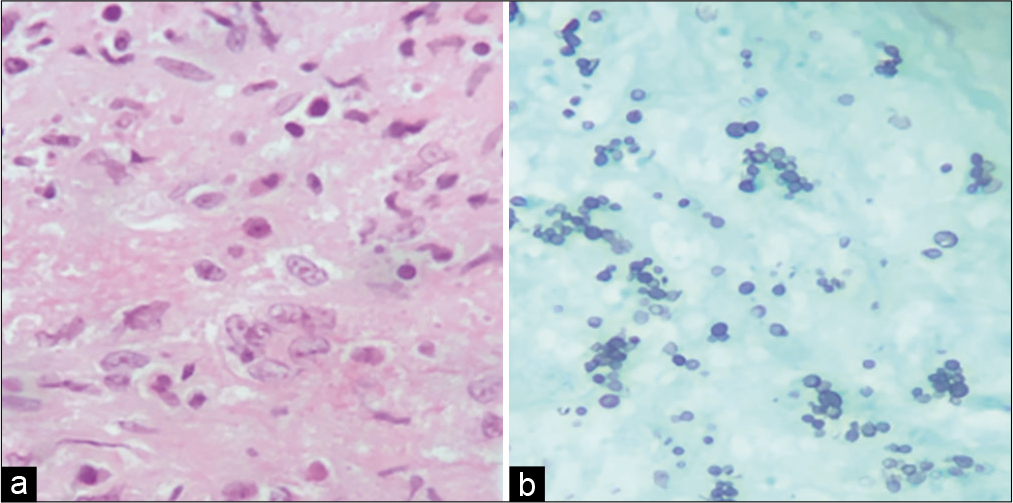
On the 20th day of hospitalization, the anatomopathological result of the spinal cord lesion compatible with histoplasmosis was obtained [
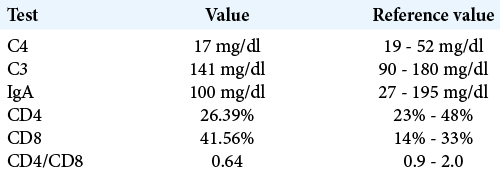
An outpatient investigation for immunodeficiencies was carried out. The HIV test was negative, but the measurement of immunoglobulin A, complement, and CD4/CD8 suggested a probable diagnosis of primary immunodeficiency [
DISCUSSION
Histoplasmosis is considered an opportunistic disease and affects mainly immunocompromised patients who are also the most susceptible to disseminated forms of the disease, including among pediatric patients.[
The most common neurological symptoms are chronic meningitis, hydrocephalus, and encephalitis, in addition to focal deficits caused by brain and spinal cord injuries.[
Diagnostic tests include analysis of serum, urine, cerebrospinal fluid, with testing for antigens and antibodies, as well as culture for fungi.[
In critically ill patients, biopsy of lesions identified on imaging studies may be preferred to repeated assessment of the CSF to obtain a more rapid definitive diagnosis or when the CSF cannot be reliably obtained.[
A recent report evaluated spinal cord involvement in histoplasmosis in adults and showed that four out of eight cases had isolated CNS involvement in the spinal cord and all of them were treated with amphotericin.[
Despite having other complications resulting from histoplasmosis (hydrocephaly), the patient in the present report evolved with significant clinical improvement after the institution of treatment with liposomal amphotericin B followed by outpatient treatment with fluconazole. After 2 years of follow-up, the patient is stable, with the previous motor sequelae and in constant rehabilitation with physiotherapy, without cognitive limitations, and with good school performance.
The differential diagnosis of patients who progress with progressive limb paresis is broad and encompasses several etiologies, including infectious causes (syphilis, fungal infections, tuberculosis, and other mycobacteria), demyelinating (acute demyelinating encephalomyelitis – ADEM), and neoplastic causes.[
The treatment recommendation for histoplasmosis in its disseminated form is different when there is involvement of the CNS. While patients without neurological involvement can be treated on an outpatient basis with itraconazole as the drug of choice, patients with neurohistoplasmosis require hospitalization and should be treated with liposomal amphotericin for 2–6 weeks, to drastically reduce fungemia followed by treatment with oral antifungal agents such as itraconazole.[
CONCLUSION
According to our research, there are no reports in the literature that address the situation of spinal cord compression syndrome due to histoplasmosis in the pediatric population.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bloch KC, Myint T, Raymond-Guillen L, Hage CA, Davis TE, Wright PW. Improvement in diagnosis of histoplasma meningitis by combined testing for histoplasma antigen and immunoglobulin G and immunoglobulin M anti-histoplasma antibody in cerebrospinal fluid. Clin Infect Dis. 2018. 66: 89-94
2. Bollyky PL, Czartoski TJ, Limaye A. Histoplasmosis presenting as an isolated spinal cord lesion. Arch Neurol. 2006. 63: 1802-3
3. Dasmasceno LS, Novaes AR, Alencar CH, Lima DT, Sidrim JJ, Gonçalves MV. Disseminated histoplasmosis and aids: Relapse and late mortality in endemic area in NorthEastern Brazil. Mycoses. 2013. 56: 520-6
4. Fischer GB, Mocelin H, Severo CB, de Mattos Oliveira F, Xavier MO, Severo LC. Histoplasmosis in children. Paediatr Respir Rev. 2009. 10: 172-7
5. Guimarães AJ, Nosanchuk JD, Zancopé-Oliveira RM. Diagnosis ofhistoplasmosis. Braz J Microbiol. 2006. 37: 1-13
6. Hage CA, Azar MM, Bahr N, Loyd J, Wheat LJ. Histoplasmosis: Up-to-date evidence-based approach to diagnosis and management. Semin Respir Crit Care Med. 2015. 36: 729-45
7. Hott JS, Horn E, Sonntag VK, Coons SW, Shetter A. Intramedullary histoplasmosis spinal cord abscess in a nonendemic region: Case report and review of the literature. J Spinal Disord Tech. 2003. 16: 212-5
8. Kelly DR, Smith CD, McQuillen MP. Successful medical treatment of a spinal histoplasmoma. J Neuroimaging. 1994. 4: 237-9
9. Manning TC, Born D, Tredway TL. Spinal intramedullary histoplasmosis as the initial presentation of human immunodeficiency virus infection: Case report. Neurosurgery. 2006. 59: E1146
10. McCarthy MW, Kalasauskas D, Petraitis V, Petraitiene R, Walsh TJ. Fungal infections of the central nervous system in children. J Pediatr Infect Dis Soc. 2017. 6: e123-33
11. Nacher M, Adenis A, McDonald S, Do Socorro Mendonca Gomes M, Singh S, Lima IL. Disseminated histoplasmosis in HIV-infected patients in South America: A neglected killer continues on its rampage. PLoS Negl Trop Dis. 2013. 7: e2319
12. Odio CM, Navarrete M, Carrillo JM, Mora L, Carranza A. Disseminated histoplasmosis in infants. Pediatr Infect Dis J. 1999. 18: 1065-8
13. Ouellette CP, Stanek JR, Leber A, Ardura MI. Pediatric histoplasmosis in an area of endemicity: A contemporary analysis. J Pediatr Infect Dis Soc. 2019. 8: 400-7
14. Recker MJ, Housley SB, Lipinski LJ. Indolent nonendemic central nervous system histoplasmosis presenting as an isolated intramedullary enhancing spinal cord lesion. Surg Neurol Int. 2021. 12: 392
15. Riddell J, Wheat LJ. Central nervous system infection with histoplasma capsulatum. J Fungi (Basel). 2019. 5: 70
16. Rivierez M, Heyman D, Brebion A, Landau-Ossondo M, Desbois N, Vally P. Spinal cord histoplasmoma. A case report. Neurochirurgie. 2002. 48: 44-8
17. Simms A, Kobayashi T, Endelman L, Sekar P. Disseminated histoplasmosis presenting as bilateral lower extremity paresis. Int J Infect Dis. 2020. 95: 265-7
18. Voelker JL, Muller J, Worth RM. Intramedullary spinal histoplasma granuloma. Case report. J Neurosurg. 1989. 70: 959-61
19. Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system. A clinical review. Medicine (Baltimore). 1990. 69: 244-60
20. Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis. 2005. 40: 844-52
21. Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am. 2016. 30: 207-27
22. Wheat J, Myint T, Guo Y, Kemmer P, Hage C, Terry C. Central nervous system histoplasmosis: Multicenter retrospective study on clinical features, diagnostic approach and outcome of treatment. Medicine (Baltimore). 2018. 97: e0245