- Department of Neurosurgery, Liaquat National Institute of Postgraduate Studies, Karachi, Pakistan.
Correspondence Address:
Fawwaz Bin Shahab, Department of Neurosurgery, Liaquat National Institute of Postgraduate Studies, Karachi, Pakistan.
DOI:10.25259/SNI_907_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Fawwaz Bin Shahab, Saad Akhtar Khan. Intramedullary schwannoma – A case report. 18-Nov-2022;13:535
How to cite this URL: Fawwaz Bin Shahab, Saad Akhtar Khan. Intramedullary schwannoma – A case report. 18-Nov-2022;13:535. Available from: https://surgicalneurologyint.com/surgicalint-articles/12011/
Abstract
Background: Schwannomas are benign but clinically progressive tumors. Mostly, they present as intradural extramedullary lesions. They are quite rare in the intramedullary (IM) region. We report a case of IM schwannoma.
Case Description: A 52-year-old gentleman presented with a history of gait instability and numbness in bilateral lower limbs. He had clinical signs of myelopathy. His magnetic resonance imaging (MRI) dorsal spine was done that showed an intradural IM lesion at the level of D11, with one differential of ependymoma. Near total resection of lesion was done and histopathology reported it schwannoma.
Conclusion: Preoperative radiologic assessment for IM spinal lesions is difficult and high degree of suspicion should be present when approaching a patient with somatic pain and IM lesion on MRI, keeping in mind one differential of IM schwannoma.
Keywords: Gross total resection, Histopathology, Intramedullary schwannoma
INTRODUCTION
Schwannomas account for 30% of primary intraspinal tumors. They are mostly intradural extramedullary, with only 1% of these reported in the literature as intramedullary (IM).[
Schwannomas usually arises from the dorsal sensory roots. Patients with extramedullary schwannoma commonly present with pain. IM Schwannomas, on the other hand, can present with wide range of symptoms. Few of these include pain, dysesthesias, and pyramidal syndrome followed by sensitivity complaints and sphincter dysfunction.[
Majority of cases of IM schwannoma have been reported in the cervical spine (63%), followed by thoracic (26%) and lumbar segments (11%) of spinal cord.[
Schwannomas originate mainly from the Schwann cells of peripheral nerve sheaths in the spinal canal and hence are rare within the spinal cord which lacks Schwann cells. The pathogenesis of IM schwannoma is still unclear and various hypothesis (listed below) has been devised that can lead to development of IM schwannoma.
IM schwannomas are picked up on magnetic resonance imaging (MRI), and their definitive diagnosis is confirmed on biopsy following surgical excision. Since they are quite rare in the literature, we report a case of IM schwannoma in a 52-year-old gentleman that was diagnosed based on MRI, followed by surgical excision and histopathology. With the advancement in MRI and preference for operative management of all IM lesions, IM schwannomas are likely to be described more often in coming years.[
CASE REPORT
We report a case study of 52-year-old gentleman with no known prior comorbids, who presented to neurosurgery clinic with complaints of frequent instability in gait and numbness in bilateral lower limbs, for around 3–4 weeks. Recently, his symptoms have worsened to an extent that he is only able to walk with support, or else he falls within 5–6 steps.
At presentation, his neurological examination revealed powers of 5/5 in both upper limbs, 5/5 in the lower limbs proximally and 4 + distally. Signs of myelopathy including brisk lower limb reflexes and bilateral upgoing plantars were noted. Sphincters were continent and no sensory impairment was appreciated.
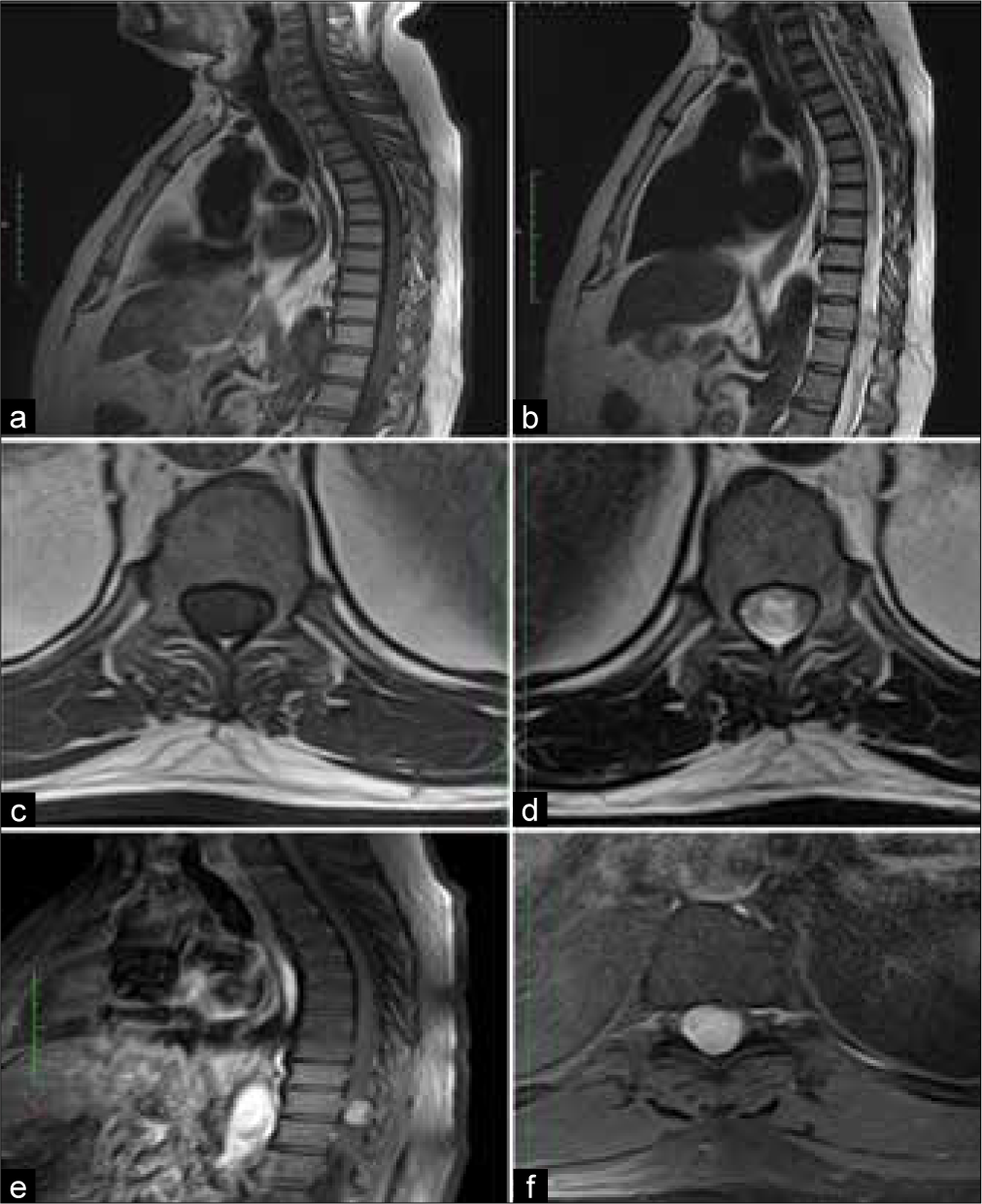
His MRI dorsolumbar spine with contrast was performed which reported an IM abnormal signal intensity area with homogenous enhancement, measuring 1.4*1.5*2.1 cm, corresponding to the level of T11, causing compression of spinal cord anteriorly and to the left side. This is associated with high signal cord edema in the lower thoracic spinal cord. No evidence of dural tail and extension in exiting foramina was noted. The lesion appears isointense on T1 and iso to hypointense on T2, with post contrast-enhancement; findings representing IM neoplasm with one differential of ependymoma [
With the impression of an IM cord tumor, patient was planned for surgical excision. After informed consent and preoperative counseling, D10 partial, D11 complete, and D12 partial laminectomy were performed.
Intraoperatively, an IM lesion was noted with no definite arachnoid planes, myelotomy was performed by the tumor itself. Hence, near total resection of tumor was done. Neuromonitoring could not be used because of unavailability.
Postoperatively, the patient had no new sensory or motor deficits in the lower limb. His active physiotherapy and limb strengthening exercises were done.
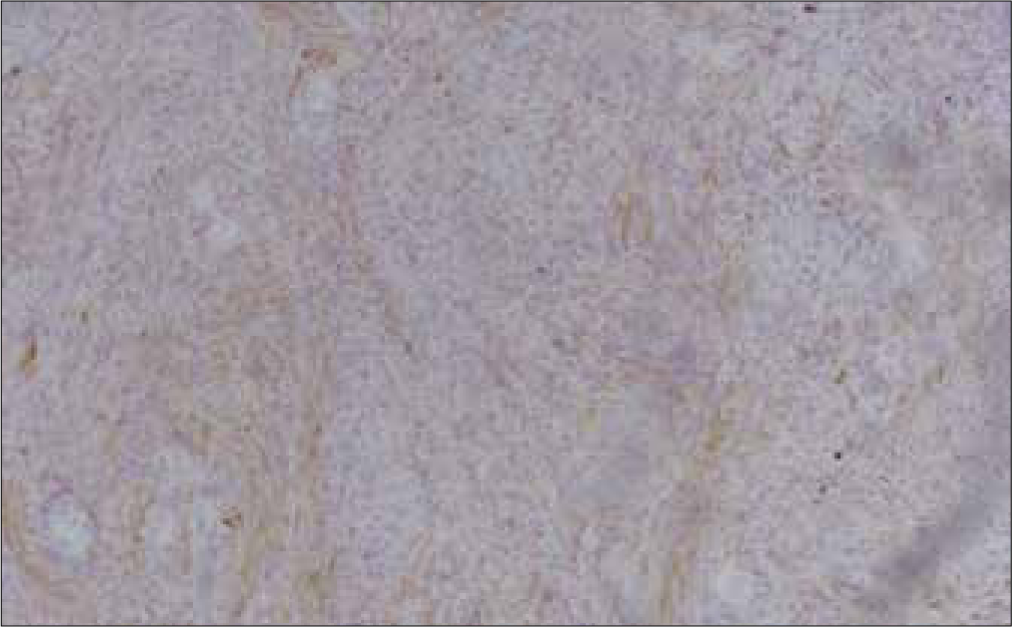
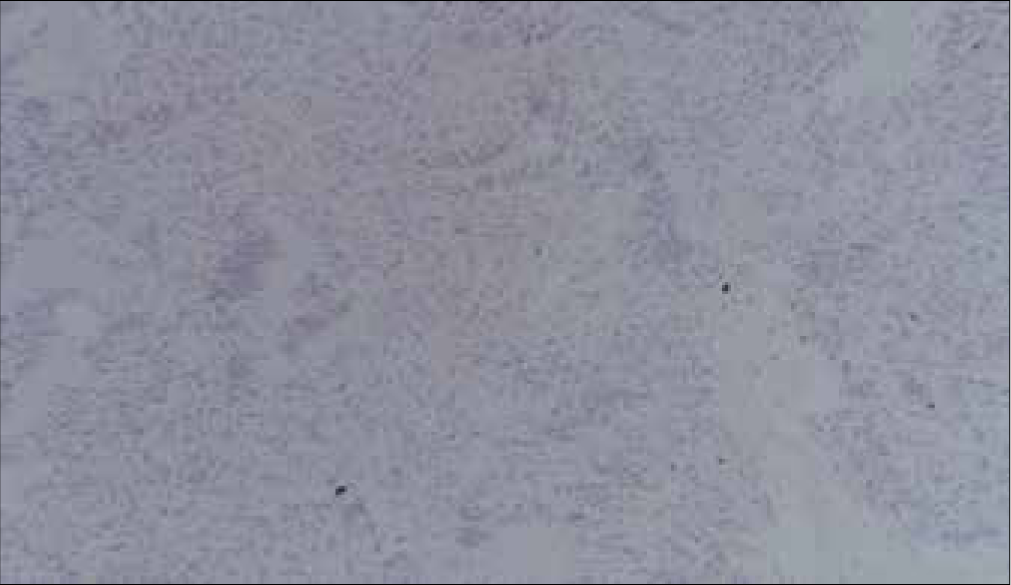
Histopathology of biopsied lesion reported areas of compact, elongated cells, with occasional nuclear palisading (Antoni A pattern) and less cellular, loosely textured cells with indistinct processes and variable lipidization (Antoni B pattern). Nuclear palisading and fibrillary processes were seen in cellular areas. Immunohistochemical stain S100 was positive in neoplastic lesion and GFAP negative in tumor cells. These findings were consistent with the diagnosis of schwannoma [
DISCUSSION
This report presents a case of 52-year-old gentleman with IM schwannoma, which is quite rare in the IM region, due to absence of Schwann cells in central nervous system. The last possible literature review was done in 2018 that quoted 70 cases of IM schwannoma reported by then.[
IM schwannomas present commonly with somatic pain and other neurologic deficits are mostly manifested later.[
Preoperative radiologic diagnosis of IM schwannoma, based on MRI alone, is challenging.[
Unlike the more malignant lesions of cord such as glioma and schwannomas are usually benign and have well defined cleavage plane.[
The accurate diagnosis of tumor is based on histopathology. To have good functional status, surgery should be performed timely before any permanent neurologic deficit develops.[
Conventional schwannomas mostly have a good prognosis, with <5% recurrence reported in the literature. However, data on long-term follow-up of IM schwannoma are lacking. Dhake and Chatterjee reported recurrence in two cases of thoracic IM schwannoma, highlighting the need for long-term follow-up in this group of patients.[
CONCLUSION
IM schwannomas are difficult to be picked up on radiologic and clinical assessment preoperatively. Since misdiagnosis may lead to inappropriate treatment[
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Conti P, Pansini G, Mouchaty H, Capuano C, Conti R. Spinal neurinomas: Retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol. 2004. 61: 34-43 discussion 44
2. Dai LM, Qiu Y, Cen B, Lv J. Intramedullary schwannoma of cervical spinal cord presenting inconspicuous enhancement with Gadolinium. World Neurosurg. 2019. 127: 418-22
3. Demachi H, Takashima T, Kadoya M, Suzuki M, Konishi H, Tomita K. MR imaging of spinal neurinomas with pathological correlation. J Comput Assist Tomogr. 1990. 14: 250-4
4. Dhake RP, Chatterjee S. Recurrent thoracic intramedullary schwannoma: Report of two cases with long term follow up. Br J Neurosurg. 2022. 36: 647-50
5. Fernández JO, Sosa AM, Díaz BC, Arrieta VA, Leyva RU, Valdez AM. Cervical intramedullary schwannoma: Case report and review of the literature. Case Rep Neurol. 2018. 10: 18-24
6. Gupta A, Nair BR, Chacko G, Mani S, Joseph V. Cervical intramedullary schwannoma mimicking a glioma. Asian J Neurosurg. 2015. 10: 42-74
7. Herregodts P, Vloeberghs M, Schmedding E, Goossens A, Stadnik T, D’Haens J. Solitary dorsal intramedullary schwannoma: Case report. J Neurosurg. 1991. 74: 816-20
8. Kelly A, Lekgwara P, Younus A. Extensive intramedullary schwannoma of the sub-axial cervical spine-a case report. Interdiscip Neurosurg. 2020. 19: 100621
9. Kodama Y, Terae S, Hida K, Chu BC, Kaneko K, Miyasaka K. Intramedullary schwannoma of the spinal cord: Report of two cases diagnostic. Neuroradiology. 2001. 43: 567-71
10. Nayak R, Chaudhuri A, Chattopadhyay A, Ghosh SN. Thoracic intramedullary schwannoma: A case report and review of literature. Asian J Neurosurg. 2015. 10: 126-8
11. Nicácio JM, Rodrigues JC, Galles MH, Faquini IV, de Brito Pereira CA, Ganau M. Cervical intramedullary schwannoma: A case report and review of the literature. Rare Tumors. 2009. 1: e44
12. WooD WG, Rothman LM. Nussbaum BE Intramedullary neurilemoma of the cervical spinal cord. J Neurosurg. 1975. 42: 465-8
13. Yang T, Wu L, Deng X, Yang C, Xu Y. Clinical features and surgical outcomes of intramedullary schwannomas. Acta Neurochir (Wien). 2014. 156: 1789-97