- Department of Neurosurgery, University of Tsukuba, Tsukuba,
- Department of Neurosurgery, Dokkyo Medical University, Shimotsuga,
- Department of Otolaryngology, University of Tsukuba, Tsukuba,
- Department of Diagnostic Radiology, Toranomon Hospital, Tokyo, Japan.
Correspondence Address:
Hiroyoshi Akutsu, Department of Neurosurgery, Dokkyo Medical University, Shimotsuga, Japan.
DOI:10.25259/SNI_473_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takaaki Ishikawa1, Hiroyoshi Akutsu2, Takuma Hara1, Shuho Tanaka3, Tomohiko Masumoto4, Eiichi Ishikawa1. Intraosseous schwannoma in the clivus mimicking chordoma treated with endoscopic endonasal surgery: A case report. 05-Aug-2022;13:346
How to cite this URL: Takaaki Ishikawa1, Hiroyoshi Akutsu2, Takuma Hara1, Shuho Tanaka3, Tomohiko Masumoto4, Eiichi Ishikawa1. Intraosseous schwannoma in the clivus mimicking chordoma treated with endoscopic endonasal surgery: A case report. 05-Aug-2022;13:346. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11758
Abstract
Background: Intraosseous schwannomas are extremely rare and they have not yet been reported to occur in the clivus. We report a schwannoma in the clivus mimicking chordoma and review intraosseous schwannomas of the skull.
Case Description: A 62-year-old man presented with gradually worsening hoarseness with dysphagia and atrophy of the left tongue, trapezius muscle, and sternocleidomastoid muscle. Magnetic resonance imaging showed that the tumor was mainly located in the clivus, and a computed tomography (CT) scan revealed an osteolytic lesion with expansion of the clivus and preservation of the bony cortex. Endoscopic endonasal surgery was performed to diagnose and treat symptoms. The tumor was subtotally removed without any complications. The histopathological findings revealed typical schwannoma, which showed Antoni A and Antoni B patterns positive for S100 protein. Based on the preoperative imaging, intraoperative and histopathological findings, the tumor was considered to be an intraosseous schwannoma in the clivus, and no recurrence was observed after 1 year of postoperative follow-up.
Conclusion: Even though the intraosseous schwannoma in the clivus is uncommon, it should be considered as a differential diagnosis if an expansive lesion without destruction of the cortical bone is shown on CT as well as iso-hyperintensity on T2-weighted magnetic resonance imaging.
Keywords: Clivus, Endoscopic endonasal surgery, Schwannoma
INTRODUCTION
Schwannomas comprise 8.6% of central nervous tumors in the Unites States,[
Here, we present the first case, to the best of our knowledge, of a schwannoma located in the clivus that was treated with endoscopic endonasal surgery (EES). We discuss the preoperative radiological diagnosis, origin, and treatment of this tumor.
CASE REPORT
A 62-year-old man presented with a 5-year history of gradually worsening hoarseness. He was referred to our department, because magnetic resonance imaging (MRI) revealed a mass lesion in his clivus. His medical history included diabetes mellitus and hyperlipidemia, and he had no remarkable family history. Neurological examination revealed dysphagia and atrophy of the left tongue, trapezius muscle, and sternocleidomastoid muscle. There were no abnormal laboratory findings, including blood counts or alkaline phosphatase levels. Laryngoscopy revealed paresis of the left vocal cord. MRI showed that the tumor was of moderately high intensity, accompanied by cystic components on T2-weighted images and heterogeneous enhancement mainly in the peripheral area with gadolinium (Gd) on T1-weighted images [
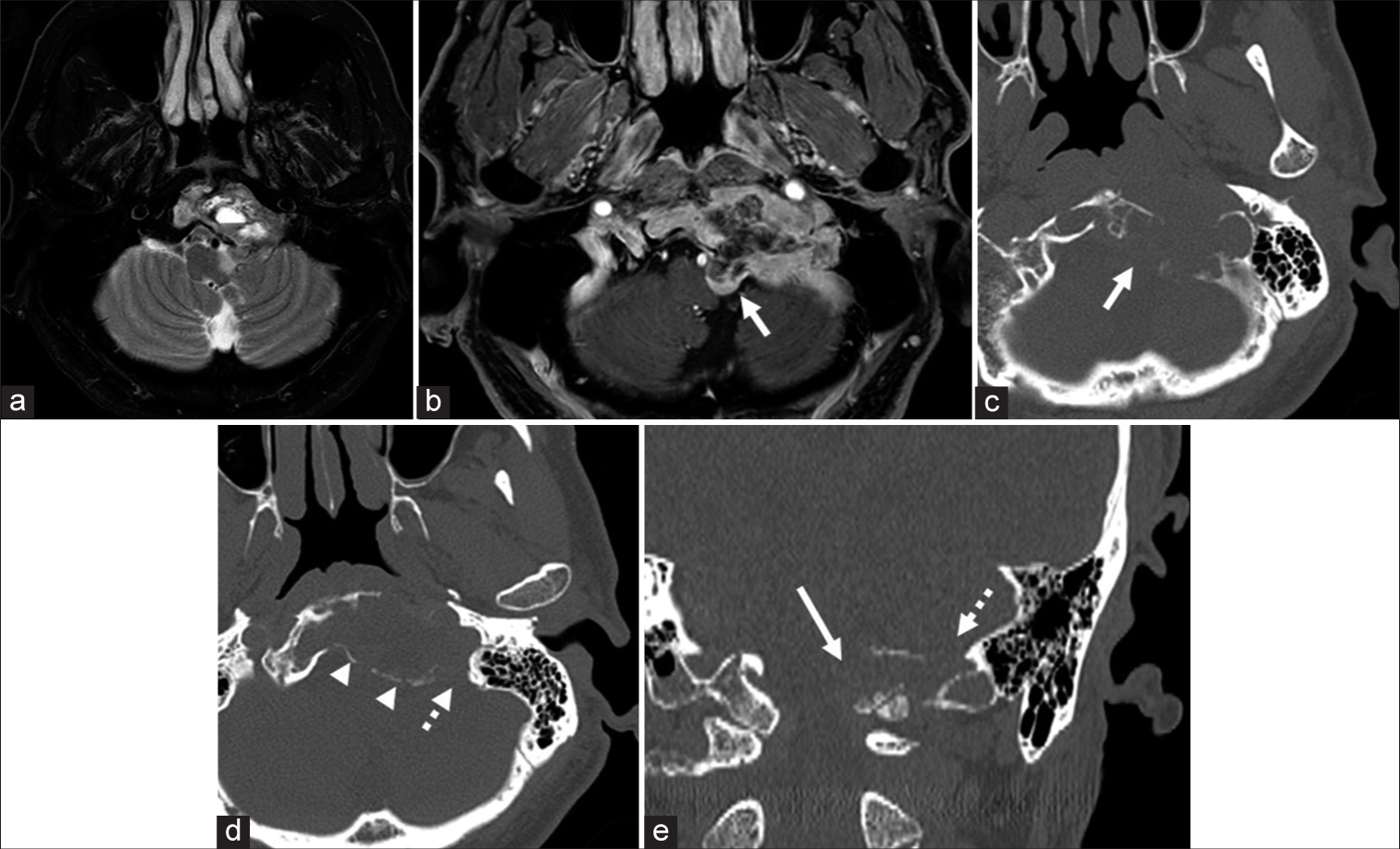
Figure 1:
Preoperative magnetic resonance imaging. Axial T2-weighted (a) and T1-weighted images with gadolinium enhancement (b) images show that the tumor is isointense with cystic components and is enhanced mainly in the peripheral area. The tumor is mainly in the clivus, and the small part of the tumor protrudes toward the brainstem around the left hypoglossal canal (the left hypoglossal canal: b,c, and e, white arrow). Axial (c and d) and coronal (e) bone window computed tomography images show destruction of the hypoglossal canal and jugular foramen (d and e, white dot arrow). The clival bone itself is expanded due to the tumor invasion (c and d), and the bony cortex is preserved in most parts (d, arrowhead).
To define the histological diagnosis, treat the patient’s symptoms, and decompress his brain stem, EES was performed using intraoperative MRI. A rapid pathological diagnosis was planned to determine the radicality of resection depending on the tumor malignancy. Nerve integrity monitoring (NIM, Medtronic, Minneapolis, MN, USA) was used to localize the lower cranial nerves (IX, XI, and XII). After dissection of the nasal septal mucosa and wide sphenoidotomy with drilling of the anterior wall, septum, and inferior wall of the sphenoid sinus, the upper part of the tumor was exposed. The tumor seemed to have no typical feature of chordoma or chondrosarcoma, rather of schwannoma. Rapid pathology of a specimen from this part of the tumor revealed no malignant findings and features of typical schwannoma. Considering the intraoperative findings and pathological result, not radical but maximum safe resection was intended. Subsequently, a left transmaxillary-transpterygoid approach with dissection of the nasopharyngeal mucosa was performed to expand the surgical field laterally and caudally. The left Vidian nerve and artery were cauterized and cut, and the contents of the left pterygopalatine fossa were lateralized to facilitate resection of the left medial pterygoid plate. The nasopharyngeal mucosa was incised in an inverted U-shape, dissected, and caudally reflected with a monopolar electrocautery to expose the lower clivus. The bone overlying the left paraclival carotid artery was removed, and the fibrocartilaginous tissue attached to the internal carotid artery (ICA) and Eustachian tube around the foramen lacerum was disconnected to enable mobility of these structures [
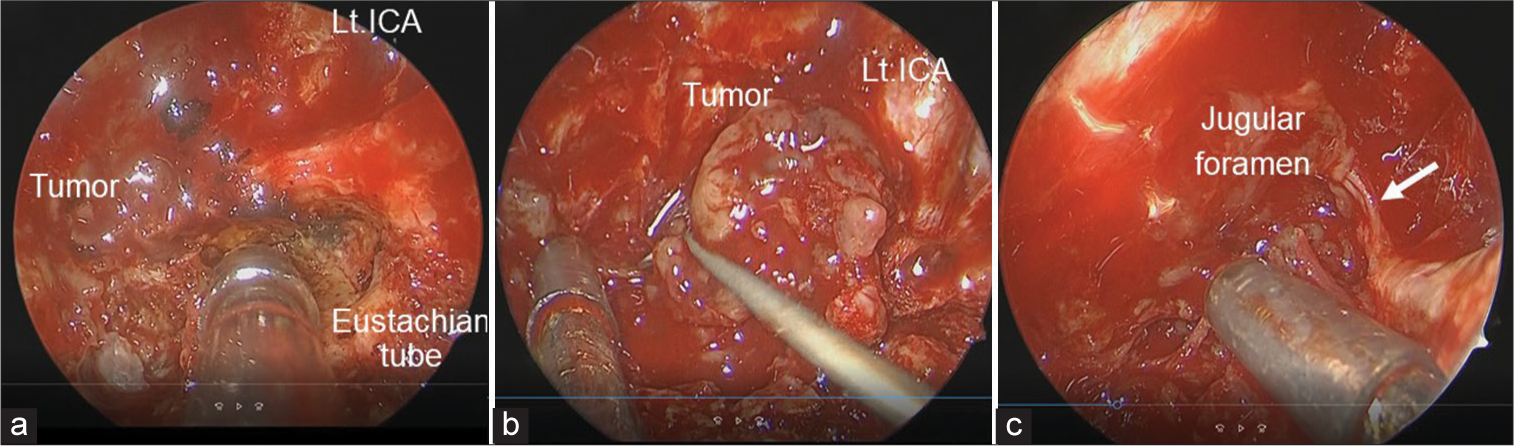
Figure 2:
Intraoperative findings of transmaxillary-pterygoid approach combined with translacerum approach. (a) Intraoperative endoscopic view showing the area around the left internal carotid artery (ICA) at the foramen lacerum. (b) The cancellous bone of the clivus is replaced by the grey-yellowish tumor. (c) Cranial nerve IX, X, or XI (white arrow) is seen at the rostral margin of the tumor around the jugular foramen.
Video 1
The histopathological findings revealed typical schwannoma, which showed spindle cells arranged in a palisading pattern with Verocay bodies (Antoni A) and a small number of tumor cells dispersed in edematous stroma (Antoni B). The tumor cells were positive for S100 protein, and the prevalence of Ki-67 positive cells was <1%. Based on the histology, preoperative MRI, and intraoperative findings, the tumor was considered an intraosseous schwannoma in the clivus.
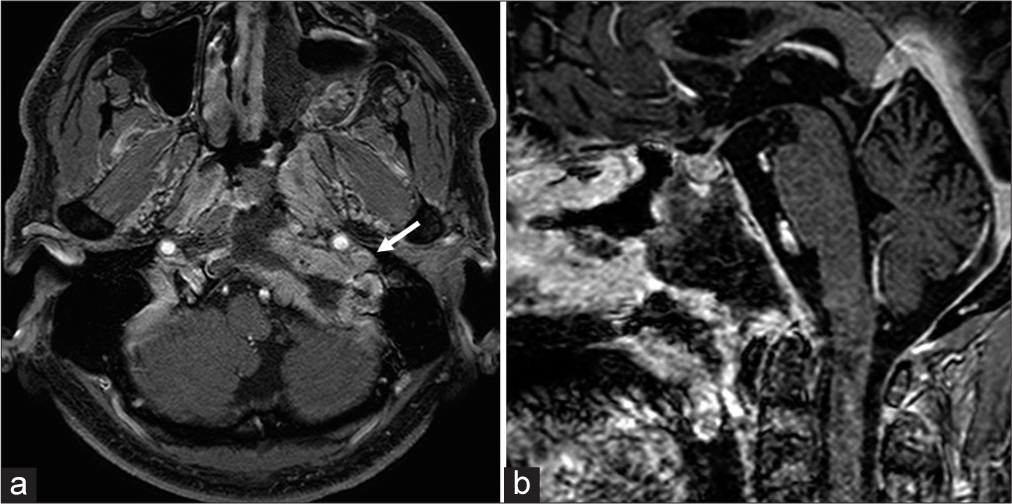
Postoperative MRI showed subtotal resection of the tumor with successful decompression of the brain stem with a small residual tumor lateral to the occipital condyle [
DISCUSSION
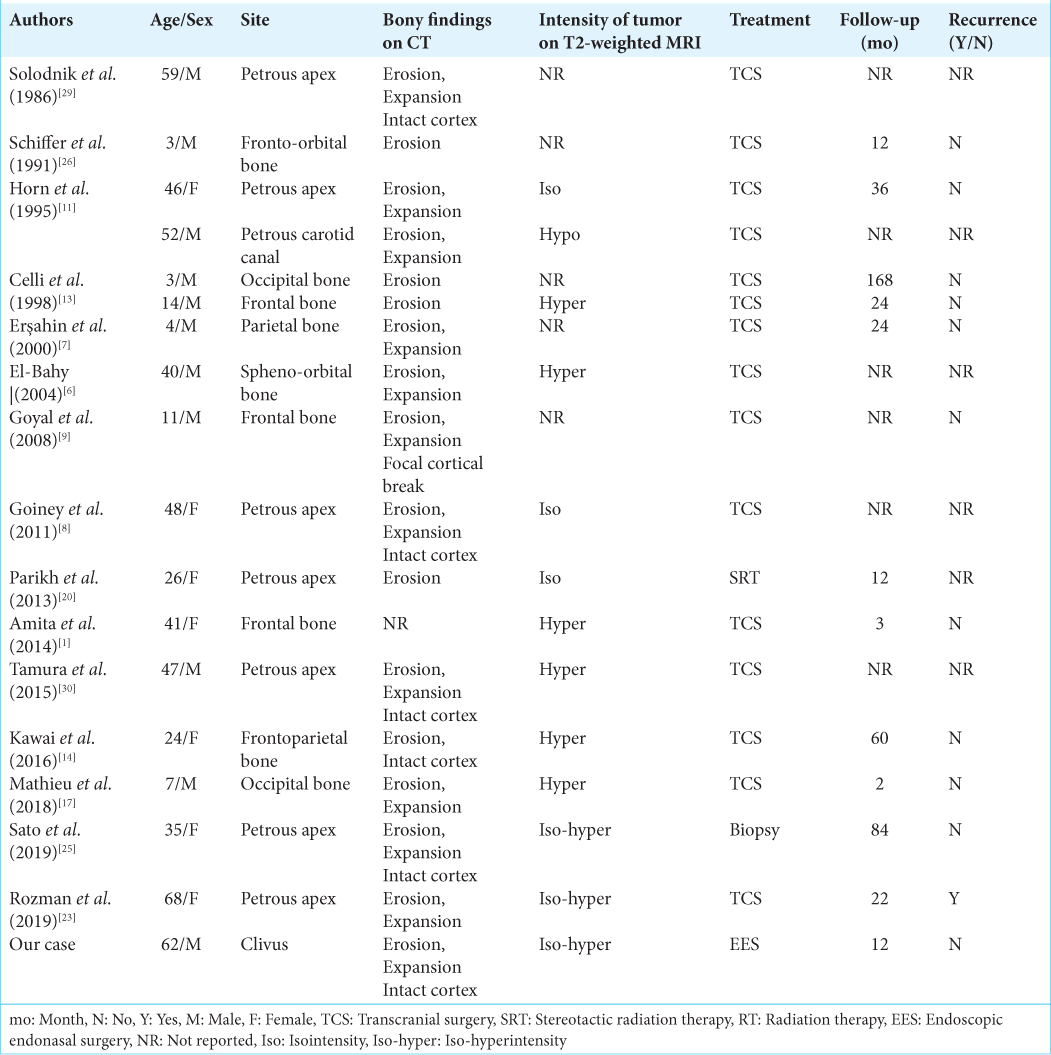
Intraosseous schwannomas are rare, because there are very few sensory nerves in bone,[
Origin of the tumor
Intraosseous schwannomas frequently occur in the mandible, sacrum, long bone, and vertebrae.[
Radiological diagnosis
Schwannomas rarely arise and extend into the clivus, and if they arise there, they mimic chordoma, which frequently arises in the clivus, as observed in our patient. The radiological differential diagnoses of clivus lesions include chondrosarcoma, fibrous dysplasia, multiple myeloma, and metastatic tumors in addition to chordoma. Chondrosarcoma frequently arises along the petro-occipital fissure and petrous bone,[
Treatment and prognosis
In our patient, intraosseous schwannomas in the clivus were successfully resected using an endoscopic endonasal transmaxillary-transpterygoid approach without any complications. Most of the reported cases of intraosseous schwannomas in the skull have been treated through craniotomy, except for one case that was treated with radiation as the first line of treatment [
Only one previously reported case with a high proliferation index (Ki-67 index, 10%) showed recurrence after surgery, and stereotactic radiation therapy was performed.[
CONCLUSION
Here, we report a case of intraosseous schwannoma in the clivus mimicking clival chordoma. The tumor was subtotally resected without any complications with EES using the transmaxillary-transpterygoid and translacerum approaches. Although an intraosseous schwannoma in the clivus is extremely rare, it should be included as one of the differential diagnoses of clivus lesions if the lesion is expanding the bone while preserving the cortex on CT images or is not hyperintense on T2-weighted MR images.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
Acknowledgment
We wish to thank the help given by Hiroyoshi Kino and Masahide Matsuda for discussing operative technique and anatomy of the area around the clivus. The authors thank Bryan J. Mathis for excellent narration of operative video and the Editage (www.editage.jp ) for English language editing.
References
1. Amita R, Sandhyamani S, Abraham M, Nair S, Praveen A, Kapilamoorthy TR. Intracalvarial schwannoma: A case report with review of literature. Neurol India. 2014. 62: 222-4
2. Bloch OG, Jian BJ, Yang I, Han SJ, Aranda D, Ahn BJ. A systematic review of intracranial chondrosarcoma and survival. J Clin Neurosci. 2009. 16: 1547-51
3. Brown EW, Megerian CA, McKenna MJ, Weber A. Fibrous dysplasia of the temporal bone: Imaging findings. AJR Am J Roentgenol. 1995. 164: 679-82
4. Celli P, Cervoni L, Colonnese C. Intraosseous schwannoma of the vault of the skull. Neurosurg Rev. 1998. 21: 158-60
5. de la Monte SM, Dorfman HD, Chandra R, Malawer M. Intraosseous schwannoma: Histologic features, ultrastructure, and review of the literature. Hum Pathol. 1984. 15: 551-8
6. El-Bahy K. Intra-osseous sphenoorbital schwannoma. Acta Neurochir (Wien). 2004. 146: 1277-8
7. Erşahin Y, Mutluer S, Demirtaş E. Intraosseous neurinoma of the parietal bone. Childs Nerv Syst. 2000. 16: 181-3
8. Goiney C, Bhatia R, Auerbach K, Norenberg M, Morcos J. Intraosseous schwannoma of the petrous apex. J Radiol Case Rep. 2011. 5: 8-16
9. Goyal R, Saikia UN, Vashishta RK, Gulati G, Sharma RK. Intraosseous schwannoma of the frontal bone. Orthopedics. 2008. 31: 281
10. Hofstetter CP, Singh A, Anand VK, Kacker A, Schwartz TH. The endoscopic, endonasal, transmaxillary transpterygoid approach to the pterygopalatine fossa, infratemporal fossa, petrous apex, and the Meckel cave. J Neurosurg. 2010. 113: 967-74
11. Horn KL, Hankinson HL, Nissen AJ, McDaniel SL. Primary schwannoma of the petrous apex. Skull Base Surg. 1995. 5: 261-8
12. Ida CM, Scheithauer BW, Yapicier O, Carney JA, Wenger DE, Inwards CY. Primary schwannoma of the bone: A clinicopathologic and radiologic study of 17 cases. Am J Surg Pathol. 2011. 35: 989-97
13. Inaoka T, Takahashi K, Hanaoka H, Aburano R, Tokusashi Y, Matsuno T. Paravertebral neurinoma associated with aggressive intravertebral extension. Skeletal Radiol. 2001. 30: 286-9
14. Kawai S, Tsugu H, Hirata Y, Abe H, Inoue T, Nabeshima K. Calvarial intraosseous schwannoma of the frontoparietal bone: A case report. No Shinkei Geka. 2016. 44: 397-402
15. Kunimatsu A, Kunimatsu N. Skull base tumors and tumor-like lesions: A pictorial review. Pol J Radiol. 2017. 82: 398-409
16. Maclean FM, Soo MY, Ng T. Chordoma: Radiological-pathological correlation. Australas Radiol. 2005. 49: 261-8
17. Mathieu F, Abel TJ, Hazrati LN, Rutka JT. Intraosseous schwannoma of the occipital bone: A case report. Childs Nerv Syst. 2018. 34: 1803-5
18. Mutlu U, Balci A, Özsan GH, Özkal S, Şeyhanli A, Özgül HA. Computed tomography characteristics of multiple myeloma and other osteolytic metastatic bone lesions. Acta Radiol. 2021. 62: 1639-47
19. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020. 22: iv1-96
20. Parikh PP, Amber KT, Angeli SI. A schwannoma of the greater petrosal nerve located within the petrous apex and treated with stereotactic radiotherapy. Am J Otolaryngol. 2013. 34: 596-9
21. Park SC, Chung SK, Choe G, Kim HJ. Spinal intraosseous schwannoma: A case report and review. J Korean Neurosurg Soc. 2009. 46: 403-8
22. Rais F, Benhmidou N, Rais G, Kouhen F, Bellahamou K, Loughlimi H. Solitary intraosseous schwannoma of the base and vault of the skull: A summary review of such unusual location. Clin Sarcoma Res. 2015. 5: 6
23. Rozman PA, Benjamin CG, Kondziolka D, Sen C, Roland JT, Zagzag D. Intraosseous petrous apex schwannoma: Case report and review of literature. World Neurosurg. 2019. 132: 182-7
24. Santegoeds RGC, Temel Y, Beckervordersandforth JC, Van Overbeeke JJ, Hoeberigs CM. State-of-the-art imaging in human chordoma of the skull base. Curr Radiol Rep. 2018. 6: 16
25. Sato M, Fujio S, Takajo T, Kamimura K, Hiraki T, Yamahata H. Large intraosseous schwannoma in petrous apex presenting with intratumoral hemorrhage. World Neurosurg. 2019. 131: 53-7
26. Schiffer J, Reif R, Lahat E, Bar-Iojai A, Starinski R. Intraosseous neurilemmoma of skull single case report. Neurochirurgia (Stuttg). 1991. 34: 178-9
27. Shidoh S, Toda M, Kawase T, Nakajima H, Tomita T, Ogawa K. Transoral vs. endoscopic endonasal approach for clival/upper cervical chordoma. Neurol Med Chir (Tokyo). 2014. 54: 991-8
28. Silveira-Bertazzo G, Manjila S, London NR, Prevedello DM. Techniques and challenges of the expanded endoscopic endonasal access to the ventrolateral skull base during the “farmedial” and “extreme medial” approaches. Acta Neurochir (Wien). 2020. 162: 597-603
29. Solodnik P, Som PM, Shugar JM, Sachdev VP, Sacher M, Lanzieri CF. Intraosseous petrous apex neuroma: CT findings. J Comput Assist Tomogr. 1986. 10: 1027-9
30. Tamura R, Takahashi S, Kohno M, Kameyama K, Fujiwara H, Yoshida K. Intraosseous schwannoma of the petrous apex. J Neurol Surg Rep. 2015. 76: e135-9
31. Taniguchi M, Akutsu N, Mizukawa K, Kohta M, Kimura H, Kohmura E. Endoscopic endonasal translacerum approach to the inferior petrous apex. J Neurosurg. 2016. 124: 1032-8
32. Uozumi Y, Taniguchi M, Umehara T, Nakai T, Kimura H, Kohmura E. Submucosal inferior turbinectomy to widen the surgical corridor for endoscopic endonasal skull base surgery. Neurol Med Chir (Tokyo). 2020. 60: 299-306
33. Vaz-Guimaraes F, Nakassa AC, Gardner PA, Wang EW, Snyderman CH, Fernandez-Miranda JC. Endoscopic endonasal approach to the ventral jugular foramen: Anatomical basis, technical considerations, and clinical series. Oper Neurosurg (Hagerstown). 2017. 13: 482-91
34. Wang YQ, Hu JX, Yang SM, Jiang L, Liu XG, Yuan HS. Intraosseous schwannoma of the mobile spine: A report of twenty cases. Eur Spine J. 2018. 27: 3092-104