- College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
- King Abdullah International Medical Research Center, Jeddah, Saudi Arabia
- Department of Medicine, Ministry of the National Guard-Health Affairs, Jeddah, Saudi Arabia
- Department of Neurosurgery, King Abdulaziz Medical City, National Guard Health Affairs, Jeddah, Saudi Arabia
- Department of Anatomical Pathology, King Abdulaziz Medical City, National Guard Health Affairs, Jeddah, Saudi Arabia.
Correspondence Address:
Moajeb Alzahrani, Department of Neurosurgery, King Abdulaziz Medical City, National Guard Health Affairs, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia.
DOI:10.25259/SNI_131_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Osama Khojah1,2,3, Sara Aljohani1,2, Abdulaziz Aldahlawi2,4, Alaa Samkari1,2,5, Moajeb Alzahrani1,2,4. Intraparenchymal meningioma in the parieto-occipital region: A case report of a diagnostically challenging tumor. 14-Apr-2023;14:135
How to cite this URL: Osama Khojah1,2,3, Sara Aljohani1,2, Abdulaziz Aldahlawi2,4, Alaa Samkari1,2,5, Moajeb Alzahrani1,2,4. Intraparenchymal meningioma in the parieto-occipital region: A case report of a diagnostically challenging tumor. 14-Apr-2023;14:135. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12259
Abstract
Background: Intraparenchymal meningioma is a rare entity of one of the most common brain tumors. It is challenging to diagnose preoperatively due to the vague clinical presentation and absence of stereotypical radiological features. These atypical features might mislead the differential to favor high-grade gliomas or brain metastasis.
Case Description: We describe a case of a 46-year-old male who presented with vertigo, right-sided sensorineural hearing loss, and bilateral blurred vision. Contrast-enhanced magnetic resonance imaging of the brain revealed a large parieto-occipital contrast-enhanced mass with a multi-loculated cystic component and diffusion restriction but without dural attachment. A gross total reaction was achieved, and the histopathological results yielded a World Health Organization Grade I meningioma diagnosis. The patient exhibited no signs of recurrence after 2 years of follow-up.
Conclusion: Intraparenchymal meningiomas are difficult to identify without histopathological assessment. We emphasize the importance of considering this diagnosis when outlining an initial differential as it may direct management planning. Total surgical resection is the best treatment modality for such cases; however, radiotherapy is a valuable option. The prognosis of intraparenchymal meningiomas is generally favorable.
Keywords: Differential diagnosis, Intraparenchymal, Meningioma, Subcortical meningioma, Transitional meningioma
INTRODUCTION
Meningiomas are the most common nonmalignant central nervous system (CNS) tumors representing 53.9% of all non-malignant cases and 37.9% of all CNS tumors.[
Brain tissue surrounding a meningioma is the defining feature of intraparenchymal meningiomas. Hence, they lack the classical signs of dural attachment on imaging.[
CASE REPORT
Presentation
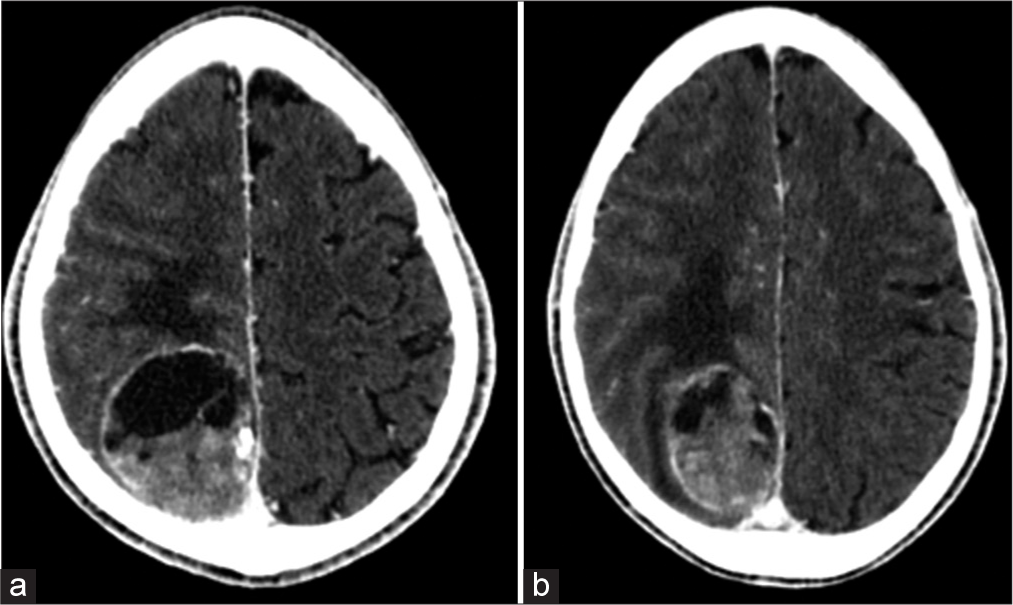
A 46-year-old male presented to the otolaryngology clinic complaining of vertigo, right-sided hearing loss, and ear discharge for 5 months. Contrast-enhanced computed tomography brain showed a parieto-occipital lesion with a well-demarcated enhanced capsule consisting of mixed solid and cystic parts [
Imaging
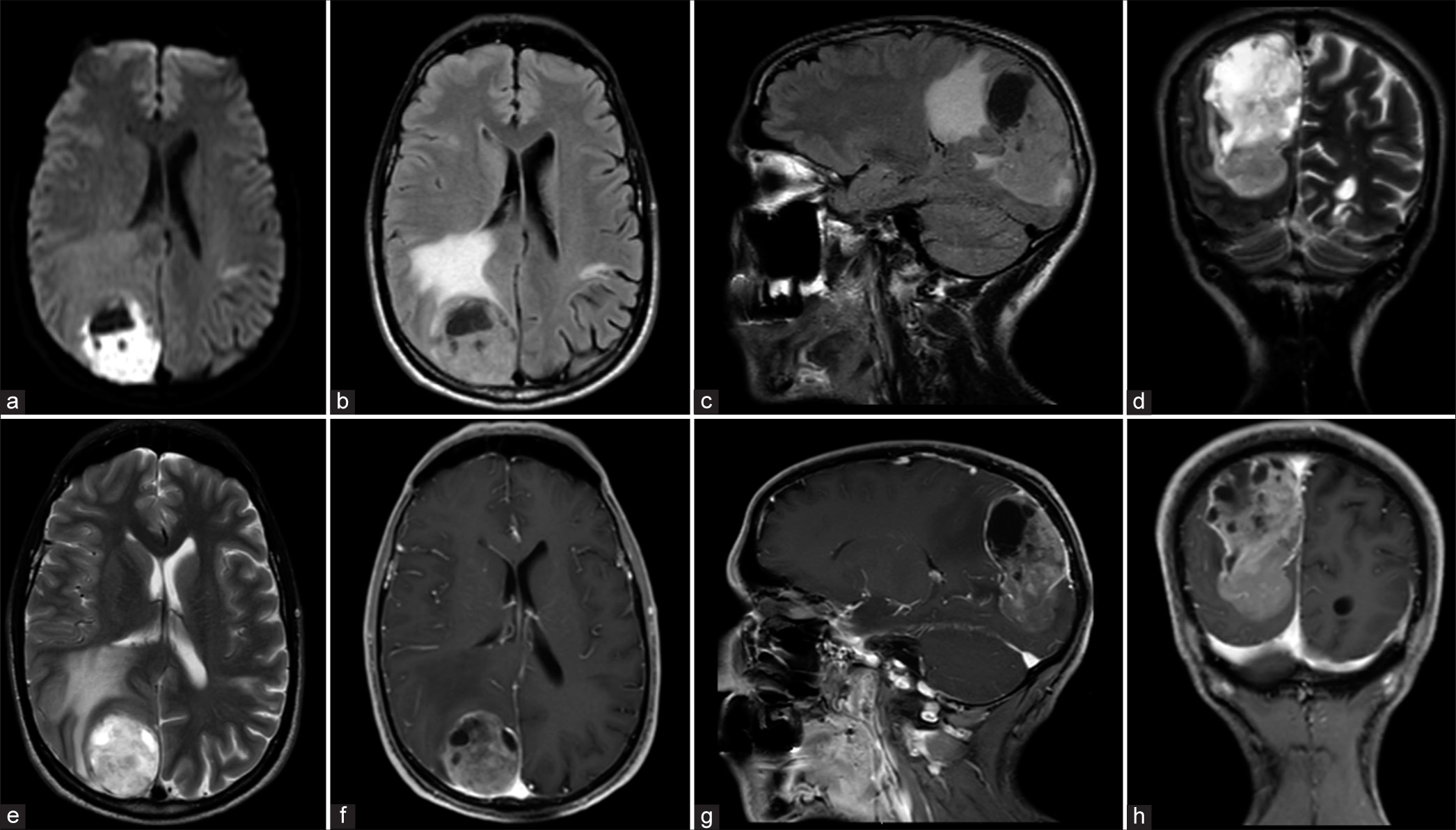
Contrast-enhanced magnetic resonance imaging (MRI) brain showed a large, complex solid, and cystic intra-axial mass centered in the right parietal and occipital lobes. Diffusion restriction was noted in the solid component and the walls of the cystic part. A large solid enhancing component was seen in the inferior and posterior margins of the mass. The cystic component had multiple loculations with a thick and irregular wall enhancement. The mass exerted a local mass effect with effacement of the cortical sulci and surrounding vasogenic edema, as well as compression on the posterior horn of the right lateral ventricle with a subtle midline shift to the left side [
Figure 2:
(A-H) Brain magnetic resonance imaging was done preoperatively. (a) axial diffusion-weighted image showing diffusion restriction in the solid part of the lesion. Fluid attenuated inversion recovery sequence images in (b) axial and (c) coronal planes, in addition to (d) coronal and (e) axial T2-weighted images showing a large solid and cystic intra-axial mass in the right parieto-occipital region with surrounding vasogenic edema. (f) axial, (g) sagittal, and (h) coronal gadolinium-enhanced T1-weighted image demonstrating an enhanced lesion and multiple loculations in the cystic component with a thick irregular enhancement of the wall.
Surgical resection
A right parietal-occipital craniotomy was performed, exposing the dura overlying the tumor, which appeared unremarkable. The lesion started to bulge out upon opening of the dura. Multiple specimens were taken for permanent histopathology. Internal debulking of the lesion started using suction and bipolar. The lesion was soft and greyish and highly vascular. However, there was a decent plane between the lesion and the surrounding parenchyma with some areas of invasion. There was no attachment to the adjacent falx cerebri, and no dural blood vessels supplying the tumor were identified intraoperatively. The lesion was then dissected circumferentially in the brain tissue following the plane, and a gross total resection was achieved.
Histopathology
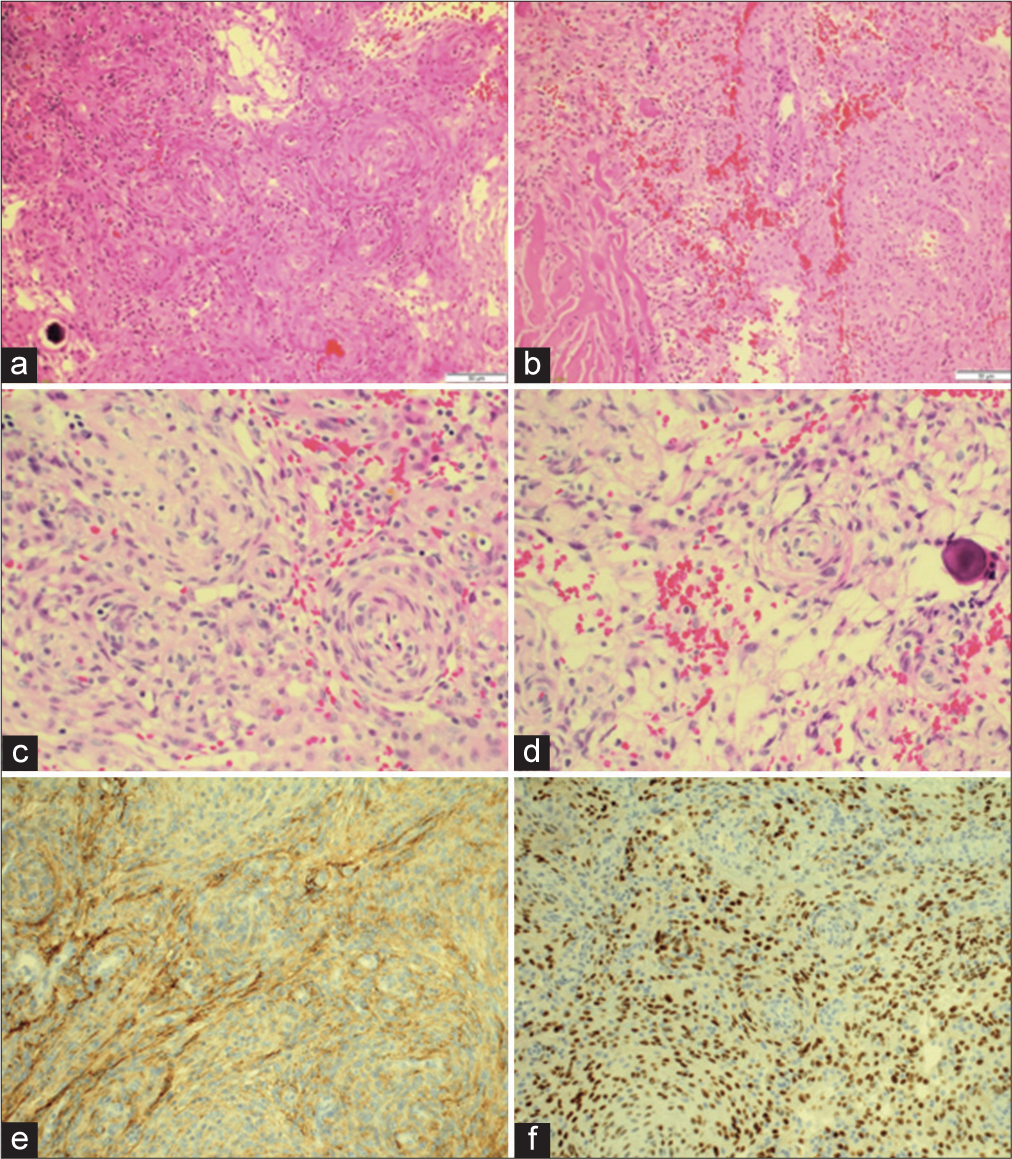
Microscopic examination of hematoxylin and eosin stained sections shows a proliferation of neoplastic cells, arranged in whorls and fascicles, with prominent vascular spaces and microcystic pattern. Psammoma bodies were identified, and no atypical features were seen. Fragments of brain parenchyma were identified without evidence of invasion by tumor cells; however, infiltration of the dura was seen. In immunohistochemistry, epithelial membrane antigen and progesterone receptor were positive. Glial fibrillary acidic protein was negative with Ki67 <2% proliferative index [
Figure 3:
Microscopic examination of hematoxylin and eosin (H and E) stained sections. (a and b) (H and E- �10) Proliferation of neoplastic meningothelial cells, infiltrating the dura. (c and d) (H and E- �20) Tumor cells are arranged in whorls, fascicles, and microcystic pattern. Psammoma bodies seen. (e) epithelial membrane antigen immunohistochemistry patchy positive staining and (f) Progesterone receptor immunohistochemistry positive nuclear staining.
Follow up
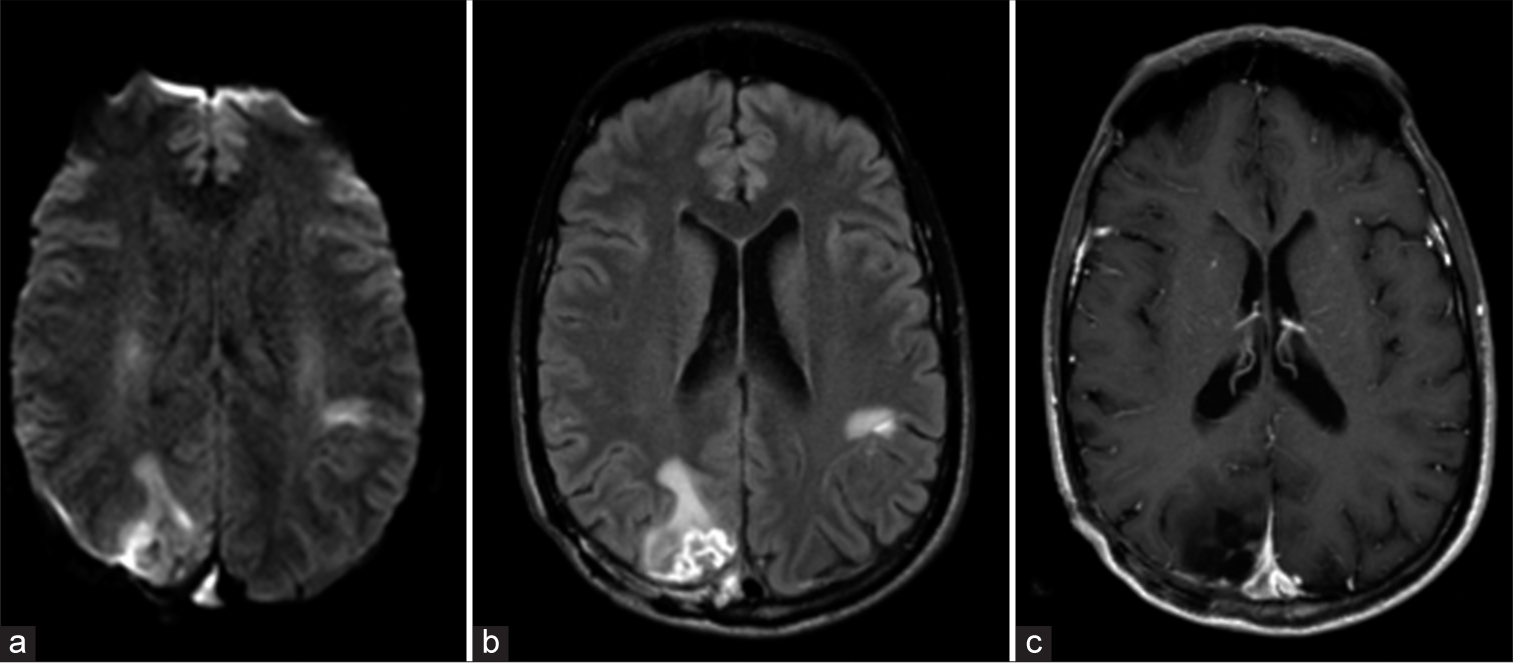
The patient’s symptoms started to improve by the 2nd day after the surgery and his headache fully resolved. Aside from the above-mentioned right-sided sensorineural hearing loss, his postoperative examination was only remarkable. MRI brain with and without contrast showed no evidence of residual tumor. On discharge, the patient had a modified Rankin scale score of one. Brain MRI with and without contrast was repeated 2–3 months later and showed no evidence of residual disease or tumor recurrence [
Figure 4:
A brain magnetic resonance imaging was done 2-month postoperatively. Axial (a) Diffusion-weighted image, (b) FLAIR, and (c) contrast-enhanced T1-weighted images showing postoperative changes noted in the right parietal and occipital lobes with areas of gliosis and focal volume loss. Nodular areas of enhancement are seen at the surgical site, consistent with scarring-no evidence of residual disease or tumor recurrence.
DISCUSSION
Meningiomas are the most common primary tumors in the CNS comprising over half of the benign brain tumors in adults.[
Unlike typical meningioma, intraparenchymal meningioma does not have a gender preference favoring females. Surprisingly, they occur more in males than females (M: F ratio, 1.9:1), while most meningiomas commonly occur more in females, with a male-to-female ratio of 1:2.5.[
Typical radiological features seen with a meningioma include a “dural tail” sign, hyperostosis, calcifications, homogeneous enhancement with contrast, and a clear demarcation between the tumor and the normal brain tissue.[
Surgical resection remains the gold standard of management for meningiomas. A gross total resection is preferable to decrease the rate of recurrence.[
CONCLUSION
The radiological features of intraparenchymal meningiomas are largely atypical of this tumor type and tend to mislead the diagnosis and steer it toward more malignant lesions. Preoperative diagnosis of this rare entity is challenging. However, it should be part of the differential diagnosis as it might direct the course of treatment and foreseen outcomes.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Almutrafi A, Bashawry Y, AlShakweer W, Al-Harbi M, Altwairgi A, Al-Dandan S. The epidemiology of primary central nervous system tumors at the national neurologic institute in Saudi Arabia: A ten-year single-institution study. J Cancer Epidemiol. 2020. 2020: 1429615
2. Curnes JT. MR imaging of peripheral intracranial neoplasms: Extraaxial vs Intraaxial masses. J Comput Assist Tomogr. 1987. 11: 932-7
3. Cushing H, Eisenhardt L. Meningiomas: Their classification, regional behavior, life history, and surgical end result. Bull Med Libr Assoc. 1938. 111: 735
4. Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH. Local control and overall survival in atypical meningioma: A retrospective study. Int J Radiat Oncol Biol Phys. 2000. 46: 57-61
5. Jadik S, Stan AC, Dietrich U, Pietilä TA, Elsharkawy AE. Intraparenchymal meningioma mimicking cavernous malformation: A case report and review of the literature. J Med Case Rep. 2014. 8: 467
6. Jia W, Sonoda Y, Saito R, Endo T, Watanabe M, Tominaga T. Intracerebral cystic rhabdoid papillary meningioma in an 11-year-old patient. Childs Nerv Syst. 2014. 30: 2151-5
7. Jiang XB, Ke C, Han ZA, Lin SH, Mou YG, Luo RZ. Intraparenchymal papillary meningioma of brainstem: Case report and literature review. World J Surg Oncol. 2012. 10: 10
8. Kotecha RS, Pascoe EM, Rushing EJ, Rorke-Adams LB, Zwerdling T, Gao X. Meningiomas in children and adolescents: A meta-analysis of individual patient data. Lancet Oncol. 2011. 12: 1229-39
9. Matsuda R, Shida Y, Nakamura M. Intraparenchymal meningioma in the basal ganglia. World Neurosurg. 2019. 128: 186-8
10. Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010. 99: 379-91
11. Miller KD, Ostrom QT, Kruchko C, Patil N, Tihan T, Cioffi G. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. 2021. 71: 381-406
12. Mut M, Söylemezoğlu F, Firat MM, Palaoğlu S. Intraparenchymal meningioma originating from underlying meningioangiomatosis. Case report and review of the literature. J Neurosurg. 2000. 92: 706-10
13. Ohba S, Abe M, Hasegawa M, Hirose Y. Intraparenchymal meningioma: Clinical, radiologic, and histologic review. World Neurosurg. 2016. 92: 23-30
14. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020. 12: iv1-96
15. Papic V, Lasica N, Jelaca B, Vuckovic N, Kozic D, Djilvesi D. Primary intraparenchymal meningiomas: A case report and a systematic review. World Neurosurg. 2021. 153: 52-62
16. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011. 115: 3-8
17. Schroeder BA, Samaraweera RN, Starshak RJ, Oechler HW. Intraparenchymal meningioma in a child: CT and MR findings. J Comput Assist Tomogr. 1987. 11: 192-3
18. Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 2015. 72: 3323-42
19. Wada T, Suzuki M, Beppu T, Arai H, Yoshida Y, Ogawa A. A case of subcortical meningioma. Acta Neurochir (Wien). 2000. 142: 209-13
20. Wasenko JJ, Hochhauser L, Stopa EG, Winfield JA. Cystic meningiomas: MR characteristics and surgical correlations. AJNR Am J Neuroradiol. 1994. 15: 1959-65
21. Watts J, Box G, Galvin A, Brotchie P, Trost N, Sutherland T. Magnetic resonance imaging of meningiomas: A pictorial review. Insights Imaging. 2014. 5: 113-22
22. Zhang J, Chi LY, Meng B, Li F, Zhu SG. Meningioma without dural attachment: Case report, classification, and review of the literature. Surg Neurol. 2007. 67: 535-9