- Department of Neurosurgery, Antonio Pedro University Hospital, Brazil.
- Department of Neurosurgery, Miguel Couto Hospital, Rio de Janeiro, Brazil.
Correspondence Address:
Othavio Gomes Lopes
Department of Neurosurgery, Antonio Pedro University Hospital, Brazil.
DOI:10.25259/SNI_526_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Othavio Gomes Lopes1, Felipe Calmon Du Pin Almeida2, Gustavo Augusto Porto Sereno Cabral1, Rodrigo Dias Guimaraes1, Ruy Castro Monteiro da Silva Filho2, Jose Alberto Landeiro1. Intraparenchymal subependymoma: Case report and literature review. 14-Apr-2021;12:154
How to cite this URL: Othavio Gomes Lopes1, Felipe Calmon Du Pin Almeida2, Gustavo Augusto Porto Sereno Cabral1, Rodrigo Dias Guimaraes1, Ruy Castro Monteiro da Silva Filho2, Jose Alberto Landeiro1. Intraparenchymal subependymoma: Case report and literature review. 14-Apr-2021;12:154. Available from: https://surgicalneurologyint.com/surgicalint-articles/10717/
Abstract
Background: Intracranial subependymomas are rare slow-growing benign tumors typically located in the ventricular system, accounting for 0.07–0.7% of all intracranial neoplasms. Intraparenchymal subependymoma is extremely rare lesions, imposing a challenging diagnosis and management.
Case Description: We describe a case of a supratentorial intraparenchymal mass on left occipital lobe in a 26-year-old woman with progressive headache and visual impairment. Differential diagnosis mainly included gliomas, neuronal-glial tumors, ependymoma, and subependymoma. Complete surgical resection was performed and histopathology analysis confirmed diagnosis of subependymoma. Despite its benign behavior the Ki67/MIB-1 labeling index assessed by immunohistochemistry was 5%. After 1 year of follow-up she was free of tumor recurrence.
Conclusion: Intraparenchymal subependymoma is extremely rare tumors and literature review showed only 11 cases reported. In general, they are misdiagnosed as other tumors, so careful attention on clinical and radiological features must be taken when looking at a tumor close to the ventricular system, even though it does not have any obvious direct connection to it. Despite its benign nature, total removal must be attempted given that there are reports of recurrence, especially in partially removed tumors with high proliferation index. The role of adjuvant therapy is still limited and new treatment options are being developed as our knowledge on biological and molecular characteristics advances.
Keywords: Atypical, Intraparenchymal, Recurrence, Subependymoma
INTRODUCTION
Subependymomas were first described by Scheinker in 1945,[
This subtype of ependymal tumors comprehend a rare slow-growing glial neoplasm, histologically corresponding as a World Health Organization (WHO) Grade I.[
Due to its silent behavior, the true incidence of subependymomas remains unclear, and they usually appear as an incidental finding on imaging or autopsies of asymptomatic patients.[
It is estimated that intracranial subependymomas account for only 0.07–0.7% of all intracranial neoplasms,[
It can be present on intracranial compartment or less frequently on the spine.[
Even less frequently, subependymomas can be present as an intraparenchymal mass presenting with focal deficits and frequently being misdiagnosed as other glial tumors. This kind of presentation can be challenging for the surgeon and lead to improper management, thus it is paramount to recognize all the features these tumors can have.
CLINICAL CASE
A 26-year-old female, previously healthy, without any remarkable medical history, presenting with left side progressive headache in the last few weeks, was admitted to the emergency department, reporting worsening of the headache and noticing visual impairment on the left eye. She was alert, calm and oriented, without other complaints. Physical examination showed right homonymous quadrantanopia on confrontation visual field testing. Fundoscopy showed no abnormalities.
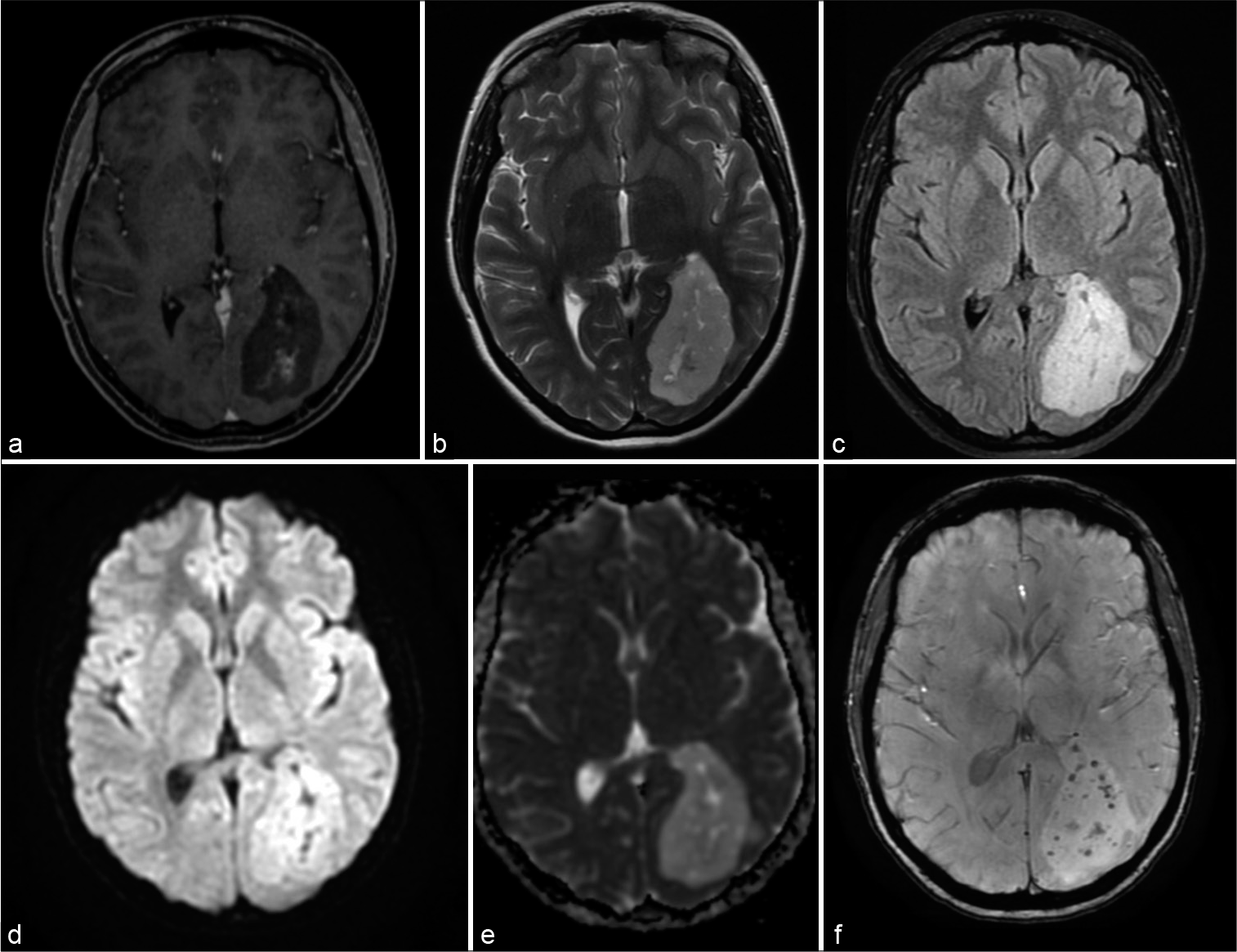
She was submitted to a computed tomography (CT) scan that revealed an intracranial hypodense mass on the left occipital lobe, measuring 5.6 × 3.8 cm. Post contrast CT was then performed and the lesion showed no enhancement. Patient, therefore, was led to a brain magnetic resonance imaging (MRI), revealing a heterogeneous intra axial mass, on the left occipital lobe, hypointense on T1-weighted sequence relative to normal gray matter, hyperintense on T2-weighted sequence relative to normal gray matter, hyperintense on FLAIR, with mild heterogeneous enhancement on central part of the lesion, and no enhancement on peripheral part of the lesion, on gadolinium T1-weighted image. Diffusion-weighted image showed no diffusion restriction while the same lesion was increased ADC relative to brain parenchyma. Susceptibility weighted imaging (SWI) showed hypointense signal dots within the lesion, corresponding to small amounts of hemorrhage. No peritumoral edema was evident. Careful analysis revealed the lesion was adjacent and related to the occipital and atrial portions of the left lateral ventricle [
Figure 1:
Imaging features of intraparenchymal subependymoma. (a) Axial gadolinium T1-weighted image showing a hypointense mass on the left occipital lobe, with mild contrast enhancement. (b) Axial T2 sequence showing a hyperintense heterogeneous mass. (c) Axial FLAIR sequence with hyperintense evident signal. (d) Axial diffusion-weighted sequence, with no diffusion restriction. (e) Axial ADC with increased signal compared to brain parenchyma. (f) Axial susceptibility weighted imaging showing small dots with hypointense signal within the lesion.
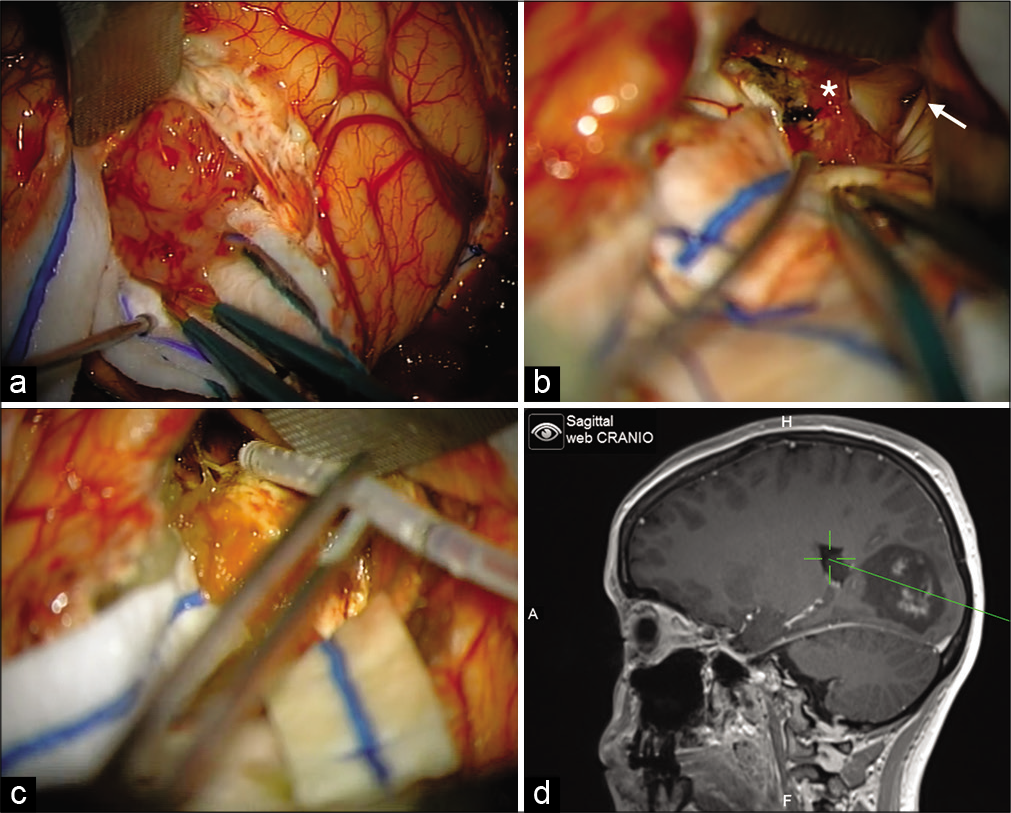
Patient was placed on a park bench position and underwent occipital craniotomy, with navigation guidance. During the final steps of the dissection a direct communication between the tumor and the ventricular system was evident, being possible to identify the occipital horn and atrium of the left lateral ventricle. After total removal, an external ventricular drainage was placed under direct view for better managing the postoperative care [
Figure 2:
Intraoperative images. (a) A 4 cm corticectomy was made and a soft, smooth-contoured, and lobulated mass was evident. (b) At the final steps of the resection it was possible to see the tumor corridor connected the walls of the ventricular system, choroid plexus (white asterisk) and the atrium of the left lateral ventricle (white arrow). (c) Ventricular drainage was placed under direct view for better managing the postoperative care. (d) Use of navigation to confirm the extent of the resection and confirming positioning of ventricular drainage.
Postoperative CT showed no signs of surgical complications. Postoperative MRI was performed on the 1st day after surgery and revealed a complete resection [
Figure 3:
Imaging of postoperative MRI. (a) Day 1 after surgery axial gadolinium T1-weighted image showing no signs of contrast enhancement or presence of hypointense lesion previously visualized. (b) Day 1 after surgery axial T2 sequence without evidence of tumor. (c) Day 1 after surgery axial FLAIR sequence with slight hyperintense signal on tumor borders, compatible with recent postoperative imaging and no evidence of residual tumor. (d) One year after surgery axial gadolinium T1-weighted image showing no signs of contrast enhancement or tumor recurrence. (e) One year after surgery axial T2 sequence without evidence of tumor recurrence. (f) One year after surgery axial FLAIR sequence without evidence of tumor recurrence and improvement on hyperintense signal on tumor borders.
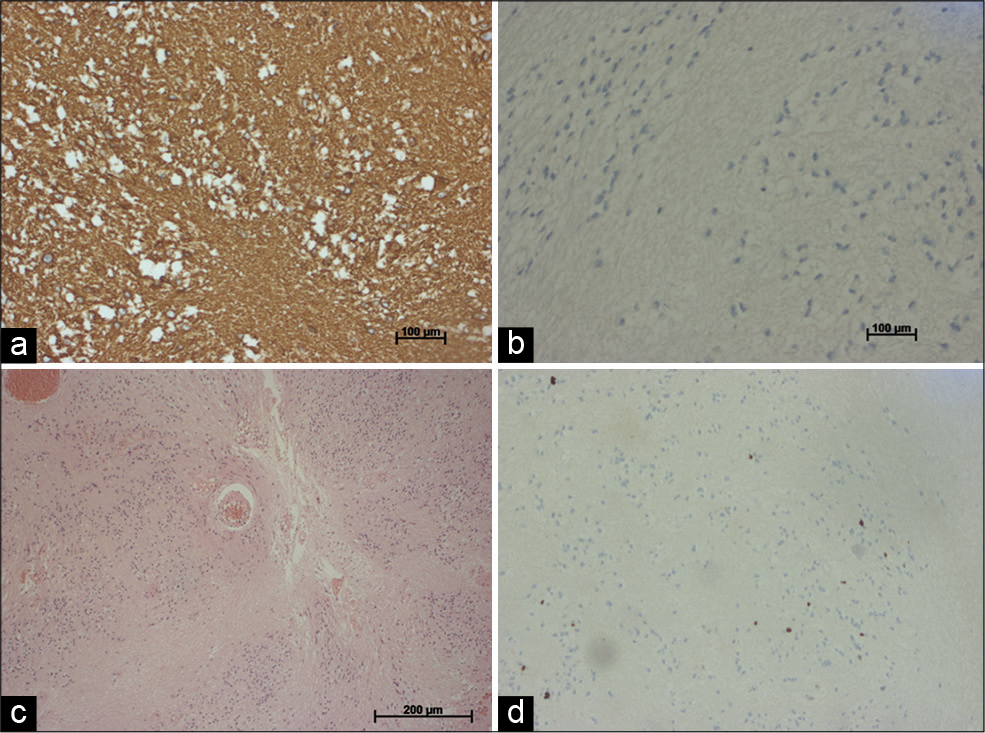
Histopathological analysis revealed hypocellular neoplasm composed of clustered cellular proliferation with round isomorphic nuclei, embedded in dense fibrillar matrix, and large acelular zones. There was no evidence of active mitosis, necrosis, or vascular endothelial proliferation [
Based on previous hypotheses and histopathological findings, immunohistochemical evaluation focused on the expression of neuronal and glial biomarkers, and revealed that the neoplastic cells were positive for glial fibrillary acidic protein (GFAP), and S100 protein. There was no immunoreactivity to epithelial membrane antigen (EMA), no loss of ATRX, no mutation on IDH1/2, and intact 1p-19q. Ki67/MIB-1 labeling index were 5% on hot spots [
Based on imaging, histopathological and immunohistochemical evaluation, the diagnosis of an intracranial subependymoma was reached.
Patient was discharged without any additional neurological deficits 5 days after surgery, referring improvement on headache and remaining with preoperative visual impairment.
One year postoperative MRI was performed on long-term follow-up, and showed no signs of recurrence [
DISCUSSION
Subependymomas account for only 0.07–0.7% of all intracranial neoplasms,[
Since the current literature regarding intracranial subependymoma is limited, its natural history and management remains unclear. Literature review showed more than 200 cases reported since first described by Scheinker in 1945, and a recent SEER-based analysis revealed a total of 466 intracranial subependymomas from 2004 to 2013, being probably the larger analysis on this kind of tumor.[
The WHO classification of CNS tumors (2016) has defined subependymomas as a subclassification of ependymomas, being considered a WHO Grade I tumor.[
Histologically, subependymomas are composed of clusters of small uniform nuclei embedded in a dense fibrillar matrix of glial cell processes associated with large acellular zones, with frequent occurrence of small cysts. Tumor nuclei are isomorphic but occasionally pleomorphic nuclei may be seen. Calcifications and hemorrhage also may be present.[
Ki67/MIB-1 labeling index is an important and well recognized marker of cell proliferation, and mitotic activity. In subependymomas, they are usually low or absent and immunohistochemical studies of MIB-1 labeling index have found proliferation values of <1%, compatible with the slow growth pattern of this entity.[
Although considered an atypical feature, some authors reported levels of Ki-67 index higher than 1%, reaching 15% in some cases.[
Immunohistochemical studies typically show immunoreactivity for neuronal markers as GFAP, neuronal cell adhesion molecule and neuron specific enolase. EMA is rarely expressed, unlike seen on ependymomas,[
On imaging they appear as a heterogeneous mass, with hypointense to isointense signal on MRI T1 sequence compared to gray matter. In the T2 sequence, they are hyperintense compared to gray matter. Contrast enhancement is generally mild to moderate. Low signal may be seen on SWI sequence, corresponding to calcifications or small hemorrhages. Peritumoral edema is typically not seen.[
The most common location of intracranial subependymomas is the fourth ventricle, accounting for 50–60% of cases, followed by lateral ventricles accounting for 30–40% of cases.[
Rarely, intracranial extraventricular sites have been reported.[
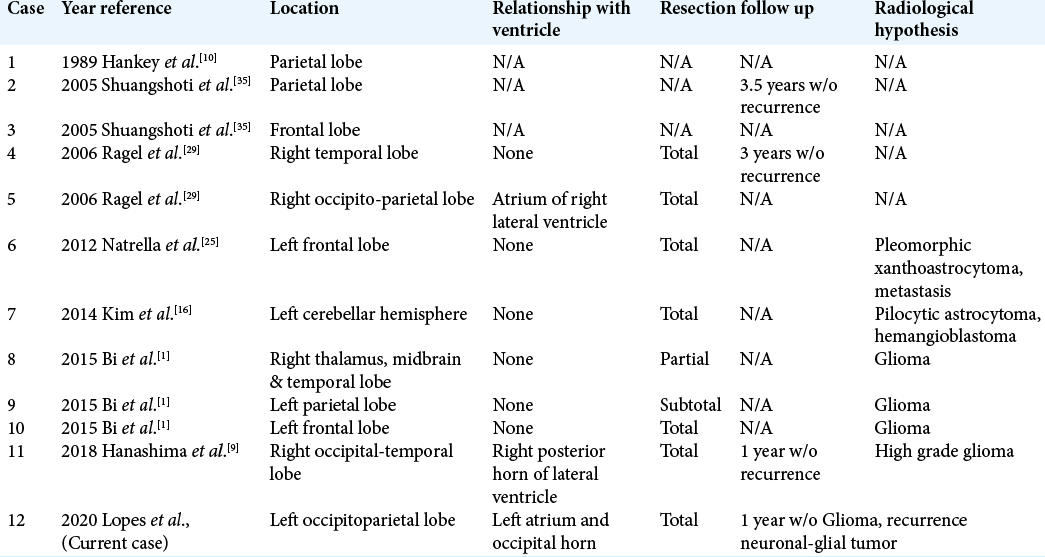
We performed a review of the English literature about subependymomas in intraparenchymal locations and found only 11 cases reported, three on case reports and eight on case series. Most cases were misdiagnosed as glial tumors prior surgery. Tumor characteristics are summarized in [
Our case had an obvious relationship to the ventricular system but there are some case reports where there are no direct connections between tumor and ventricular ependyma.[
Another interesting fact is that most subependymomas occur in the fourth ventricle; however, there is only one case report of intraparenchymal cerebellar subependymoma.[
Differential diagnosis is usually made between typical tumors of ventricular system, such as ependymomas, medulloblastomas, central neurocytomas, astrocytomas, and meningiomas.[
There are no typical radiological features that allow us to elucidate the differential diagnosis between these tumors and gliomas, neuronal-glial tumors, or other ependymal tumors; therefore, histopathological and immunohistochemical analysis is focused on this fact.
From our literature review, authors with intraparenchymal subependymomas report difficulty in distinguishing it from other tumors and patients underwent surgery with other diagnostic hypothesis such as gliomas, pilocytic astrocytoma, hemangioblastoma, pleomorphic xanthoastrocytoma, and metastasis [
Classical management of symptomatic subependymomas is surgery with maximum safe tumor resection.[
Some authors argue in favor of surgery even in asymptomatic cases because of the good general outcome after surgical resection, potential surgical cure, and avoiding the risk of clinical and neurological deterioration. In most series, surgery for subependymoma carries a good outcome, with gross total resection rates >70% and good outcomes after surgery.[
There are reports of tumor recurrence after subtotal removal, especially in tumors with atypical high Ki67/MIB-1 labeling index.[
Today, we still do not have enough data to elucidate this question but it seems that recurrence rates are low but it can happen, even after gross total removal, especially in patients with high levels of ki-67 proliferation index.
Radiotherapy is reported for treatment of recurrent or residual tumors but the true role in the management of these lesions is still unclear.[
There are some reports of radiotherapy with good results, but the limited number of patients and follow-up period preclude any conclusions. Statistical analysis of the SEER database showed that the use of radiation therapy is not a significant prognostic factor in treatment of subependymomas,[
Molecular, immune and genetic studies have gained great prominence in the neuro-oncological field, trying to discover more effective and less harmful treatment methods. A study of potential therapeutic targets reported that TOP2B, HIF1-alpha, MDM2, nucleolin, and phosphorylated STAT3 are frequently expressed in subependymomas, and also that a topoisomerase inhibitor and a p-STAT3/HIF-1α inhibitor, demonstrated a growth inhibition of the subependymoma cell proliferation.[
This data suggest that these agents may have clinical impact in subependymoma treatment, but further research is necessary to reach any conclusions.
CONCLUSION
Intraparenchymal subependymomas are extremely rare tumors, generally misdiagnosed as other tumors before surgery, so careful attention on clinical and radiological features must be taken when looking at a tumor close to the ventricular system.
They usually have a benign nature, with low Ki67/MIB-1 labeling index, but high levels of proliferation index may be found and seems to have impact on tumor recurrence, notably on subtotally removed tumors.
Surgical management is usually recommended, and due to the possibility of cure, total removal must be attempted. New adjuvant treatment options are being developed as our knowledge on biological and molecular characteristics advances, but to this date there are no proven effective adjuvant therapy on this type of tumor.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Dr. Ericka Galhardi for her excellent technical assistance.
References
1. Bi Z, Ren X, Zhang J, Jia W. Clinical, radiological, and pathological features in 43 cases of intracranial subependymoma. J Neurosurg. 2015. 122: 49-60
2. Brown DF, Rushing EJ. Subependymomas: Clinicopathologic study of 14 tumors. Arch Pathol Lab Med. 1999. 123: 873
3. Chiechi MV, Smirniotopoulos JG, Jones RV. Intracranial subependymomas: CT and MR imaging features in 24 cases. Am J Roentgenol. 1995. 165: 1245-50
4. D’Amico RS, Praver M, Zanazzi GJ, Englander ZK, Sims JS, Samanamud JL. Subependymomas are low-grade heterogeneous glial neoplasms defined by subventricular zone lineage markers. World Neurosurg. 2017. 107: 451-63
5. Ecker RD, Pollock BE. Recurrent subependymoma treated with radiosurgery. Stereotact Funct Neurosurg. 2004. 82: 58-60
6. Ellison DW, Aldape KD, Capper D, Fouladi M, Gilbert MR, Gilbertson RJ. cIMPACT-NOW update 7: Advancing the molecular classification of ependymal tumors. Brain Pathol. 2020. 30: 863-6
7. Ernestus RI, Schröder R. Clinical aspects and pathology of intracranial subependymoma-18 personal cases and review of the literature. Neurochirurgia. 1993. 36: 194-202
8. Fujisawa H, Hasegawa M, Ueno M. Clinical features and management of five patients with supratentorial subependymoma. J Clin Neurosci. 2010. 17: 201-4
9. Hanashima Y, Homma T, Maebayashi T, Igarashi T, Ishige T, Hao H. A symptomatic large subependymoma with neuroradiological features mimicking a high-grade glioma: A case report. Neurocirugia. 2019. 30: 193-7
10. Hankey GJ, Davies L, Gubbay SS. Long term survival with early childhood intracerebral tumours. J Neurol Neurosurg Psychiatry. 1989. 52: 778-81
11. Ildan F, Cetinalp E, Bagdatoglu H, Tunah N, Gönlüşen G, Karadayi A. Surgical treatment of symptomatic subependymoma of the nervous system. Report of five cases. Neurosurg Rev. 1994. 17: 145-50
12. Jain A, Amin AG, Jain P, Burger P, Jallo GI, Lim M. Subependymoma: Clinical features and surgical outcomes. Neurol Res. 2012. 34: 677-84
13. Jallo GI, Zagzag D, Epstein F. Intramedullary Subependymoma of the spinal cord. Neurosurgery. 1996. 38: 251-7
14. Kammerer S, Mueller-Eschner M, Lauer A, Luger AL, Quick-Weller J, Franz K. Subependymomas characteristics of a “leave me alone” lesion. Röfo. 2018. 190: 955-66
15. Kandenwein JA, Bostroem A, Feuss M, Pietsch T, Simon M. Surgical management of intracranial subependymomas. Acta Neurochir. 2011. 153: 1469-75
16. Kim Y, Lee SY, Yi KS, Cha SH, Gang MH, Cho BS. Infratentorial and intraparenchymal subependymoma in the cerebellum: case report. Korean J Radiol. 2014. 15: 151-5
17. Kong LY, Wei J, Haider AS, Liebelt BD, Ling X, Conrad CA. Therapeutic targets in subependymoma. J Neuroimmunol. 2014. 277: 168-75
18. Koutourousiou M, Georgakoulias N, Kontogeorgos G, Seretis A. Subependymomas of the lateral ventricle: Tumor recurrence correlated with increased Ki-67 labeling index. Neurol India. 2009. 57: 191-3
19. Kurian KM, Jones DT, Marsden F, Openshaw SW, Pearson DM, Ichimura K. Genome-wide analysis of subependymomas shows underlying chromosomal copy number changes involving chromosomes 6, 7, 8 and 14 in a proportion of cases. Brain Pathol. 2008. 18: 469-73
20. Laxton AW, Shannon P, Nag S, Farb RI, Bernstein M. Rapid expansion of a previously asymptomatic subependymoma. Case report. J Neurosurg. 2005. 103: 1084-7
21. Lombardi D, Scheithauer BW, Meyer FB, Forbes GS, Shaw EG, Gibney DJ. Symptomatic subependymoma: A clinicopathological and flow cytometric study. J Neurosurg. 1991. 75: 583-8
22. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization Classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
23. Maiuri F, Gangemi M, Iaconetta G, Signorelli F, De Caro MD. Symptomatic subependymomas of the lateral ventricles. Report of eight cases. Clin Neurol Neurosurg. 1997. 99: 17-22
24. Matsumura A, Ahyai A, Hori A, Schaake T. Intracerebral subependymomas. Clinical and neuropathological analyses with special reference to the possible existence of a less benign variant. Acta Neurochir. 1989. 96: 15-25
25. Natrella F, Mariottini A, Rocchi R, Miracco C. Supratentorial neurenteric cyst associated with a intraparenchymal subependymoma. BMJ Case Rep. 2012. 2012: bcr0120125566
26. Nguyen HS, Doan N, Gelsomino M, Shabani S. Intracranial subependymoma: A SEER analysis 2004-2013. World Neurosurg. 2017. 101: 599-605
27. Nishimura H, Fukami S, Endo K, Suzuki H, Sawaji Y, Seki T. A case of rapidly-progressing cervical spine subependymoma with atypical features. Spine Surg Relat Res. 2019. 3: 91-4
28. Prayson RA, Suh JH. Subependymomas: Clinicopathologic study of 14 tumors, including comparative MIB-1 immunohistochemical analysis with other ependymal neoplasms. Arch Pathol Lab Med. 1999. 123: 306-9
29. Ragel BT, Osborn AG, Whang K, Townsend JJ, Jensen RL, Couldwell WT. Subependymomas: An analysis of clinical and imaging features. Neurosurgery. 2006. 58: 881-90
30. Rushing EJ, Cooper PB, Quezado M, Begnami M, Crespo A, Smirniotopoulos JG. Subependymoma revisited: Clinicopathological evaluation of 83 cases. J Neurooncol. 2007. 85: 297-305
31. Scheinker IM, Scheinker IM. Subependymoma: A newly recognized tumor of subependymal derivation. J Neurosurg. 1945. 2: 232-40
32. Scheithauer BW. Symptomatic subependymoma. Report of 21 cases with review of the literature. J Neurosurg. 1978. 49: 689-96
33. Schiffer D, Chiò A, Giordana MT, Migheli A, Palma L, Pollo B. Histologic prognostic factors in ependymoma. Childs Nerv Syst. 1991. 7: 177-82
34. Seol HJ, Hwang SK, Choi YL, Chi JG, Jung HW. A case of recurrent subependymoma with subependymal seeding: Case report. J Neurooncol. 2003. 62: 315-20
35. Shuangshoti S, Rushing EJ, Mena H, Olsen C, Sandberg GD. Supratentorial extraventricular ependymal neoplasms: A clinicopathologic study of 32 patients. Cancer. 2005. 103: 2598-605
36. Spoto GP, Press GA, Hesselink JR, Solomon M.editors. Intracranial ependymoma and subependymoma: MR manifestations. Am J Roentgenol. 1990. 154: 837-45
37. Tiwari N, Powell SZ, Takei H. Recurrent subependymoma of fourth ventricle with unusual atypical histological features: A case report. Pathol Int. 2015. 65: 438-42
38. Varma A, Giraldi D, Mills S, Brodbelt AR, Jenkinson MD. Surgical management and long-term outcome of intracranial subependymoma. Acta Neurochir. 2018. 160: 1793-9
39. Vitanovics D, Áfra D, Nagy G, Hanzely Z, Turányi E, Banczerowski P. Symptomatic subependymomas of the ventricles. Review of twenty consecutive cases. Ideggyogy Sz. 2014. 67: 415-9
40. Wang Z, Hou Z, Zhang J, Tian R, Wu Z, Zhang L. Lateral ventricular subependymomas: An analysis of the clinical features of 27 adult cases at a single institute. Neurol India. 2012. 60: 379
41. You H, Kim YI, Im SY, Suh-Kim H, Paek SH, Park SH. Immunohistochemical study of central neurocytoma, subependymoma, and subependymal giant cell astrocytoma. J Neurooncol. 2005. 74: 1-8
42. Zenmyo M, Ishido Y, Terahara M, Yamamoto T, Tanimoto A, Komiya S. Intramedullary subependymoma of the cervical spinal cord: A case report with immunohistochemical study. Int J Neurosci. 2010. 120: 676-9
43. Zhou S, Xiong J, Pan J, Zhang T, Geng D, Zhang J. Neuroradiological features of cervical and cervicothoracic intraspinal subependymomas: A study of five cases. Clin Radiol. 2016. 71: 499.e9-15