- Section of Neurosurgery, The Memon Medical Institute, Karachi, Pakistan
- Section of Neurosurgery, The Aga Khan University Hospital, Karachi, Pakistan
- Department of Medicine, The Aga Khan University Hospital, Karachi, Pakistan
Correspondence Address:
Muhammad Shahzad Shamim
Department of Medicine, The Aga Khan University Hospital, Karachi, Pakistan
DOI:10.4103/sni.sni_81_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Saad Akhtar Khan, Muhammad Waqas, Usman T. Siddiqui, Muhammad Shahzad Shamim, Karim Rizwan Nathani, Rashid Jooma, Faisal Mehmood. Intrathecal and intraventricular antibiotics for postoperative Gram-negative meningitis and ventriculitis. 26-Sep-2017;8:226
How to cite this URL: Saad Akhtar Khan, Muhammad Waqas, Usman T. Siddiqui, Muhammad Shahzad Shamim, Karim Rizwan Nathani, Rashid Jooma, Faisal Mehmood. Intrathecal and intraventricular antibiotics for postoperative Gram-negative meningitis and ventriculitis. 26-Sep-2017;8:226. Available from: http://surgicalneurologyint.com/surgicalint-articles/intrathecal-and-intraventricular-antibiotics-for-postoperative-gram%e2%80%91negative-meningitis-and-ventriculitis/
Abstract
Background:Postoperative meningitis is a growing cause of concern, especially with the evolution of multidrug-resistant organism. The authors evaluate the use of intraventricular/intrathecal (IVT/IT) antibiotics for postoperative gram-negative meningitis in patients whom intravenous antibiotics were ineffective.
Methods:Medical records were retrospectively reviewed and neurosurgery patients with gram-negative postoperative infection meningitis/ventriculitis were enrolled in the study. Their demographics, hospital course, and outcomes were recorded in a pro forma and analyzed using Statistical Package for the Social Sciences, version 19.
Results:The review identified 21 patients with postneurosurgical gram-negative meningitis/ventriculitis who were treated with IVT or IT antibiotics. The most common organism was Acinetobacter species (n = 14; 66%). Amikacin was used in 7 patients, polymyxin B in 9 patients, and colistin in 5 patients. A combination of antibiotics was used in one patient. Cerebrospinal fluid sterility was achieved in all patients with no incidence of relapse. There was a single death, though that was not related to the infectious process as the patient had a massive pulmonary embolism.
Conclusion:The findings of this study suggest that IVT and IT antibiotic therapy is a useful option in patients who are nonresponsive to standard intravenous therapy with little or no side effects.
Keywords: Antibiotics, gram negative, intrathecal, intraventricular, meningitis, postoperative, ventriculitis
INTRODUCTION
Postoperative meningitis and ventriculitis are one of the grave delayed complications of neurosurgical procedures. Recently, an increase in incidence of postoperative meningitis has been observed.[
Intraventricular (IVT) and intrathecal (IT) antibiotics were reported to be used as back as a century ago.[
The use of IVT and IT antibiotic therapies has recently regained interest. An earlier infantile randomized control trial identified a three-fold increased relative risk of mortality with the use of IVT/IT and intravenous[
Most papers addressing this very important neurosurgical topic share the common limitations of small sample size, heterogeneity of patient population, and treatment plans.[
MATERIALS AND METHODS
The Aga Khan University Hospital is an ISO certified tertiary care hospital with Joint Commission International Accreditation (JCIA) and established Neurosciences program. The hospital maintains a vigorous medical recording system based on electronic as well as manually compiled patient records, logged through International Classification of Diseases (ICD) coding. We reviewed our medical records for all patients admitted to the hospital from January 2008 to December 2012, with a history of neurosurgical procedure performed within 2 weeks of presentation and later presenting with “culture proven” gram-negative ventriculitis/meningitis. Patients who responded to IV antibiotic therapy alone, not requiring IVT or IT antibiotics were excluded. The study was exempted by the Ethics Review Committee as it was a retrospective chart review.
IV antibiotics were started in all patients as soon as a diagnosis was made on cerebrospinal fluid (CSF) analysis. All patients underwent CSF analysis once at least every third day until the CSF culture was negative or the patient was discharged. Patients were started on IVT/IT antibiotics only in cases where IV antibiotics were ineffective despite 5 days of IV therapy, or if the first CSF analysis showed frank pus. IVT/IT antibiotics were administered either through EVD or LD. Dosages were based on available literature on the respective minimum inhibitory concentrations (MICs). IVT/IT antibiotics were continued until three consecutive CSF cultures were negative.
Data were collected on a standardized pro forma for all relevant clinical, laboratory, and radiological variables. Data were analyzed on Statistical Package for the Social Sciences, version 19 (SPSS IBM v 19). Continuous variables with normal distribution were expressed with mean and standard deviation, while continuous data without normal distribution were expressed with median and range. Categorical data were expressed as percentage and proportions. Mann–Whitney U-test was applied to compare nonparametric numerical data. Categorical data were compared using Chi-square test. A P value of 0.05 was regarded as significant.
Biochemical outcomes were based on the concepts of cure, relapse, and failure. “Cure” was defined as three consecutive negative CSF culture results and no relapse after withdrawal of antibiotics; “Relapse” was defined as isolation of the same organism from the CSF or central nervous system (CNS) lesion within 3 weeks of completing therapy; and “Failure” was defined as no biochemical response to treatment. Clinical outcomes were reported as pretreatment and posttreatment Glasgow Coma Scale (GCS) score. We further classified the standard GCS.[
RESULTS
During the study period, 94 cases were reported of postoperative meningitis/ventriculitis at our institution. Of these, 63 were culture proven gram positive and hence excluded from our study. The remaining 31 patients either had isolated gram-negative meningitis/ventriculitis or mixed gram-positive/negative infection, with 21 patients out of these requiring treatments with IVT/IT antibiotics.
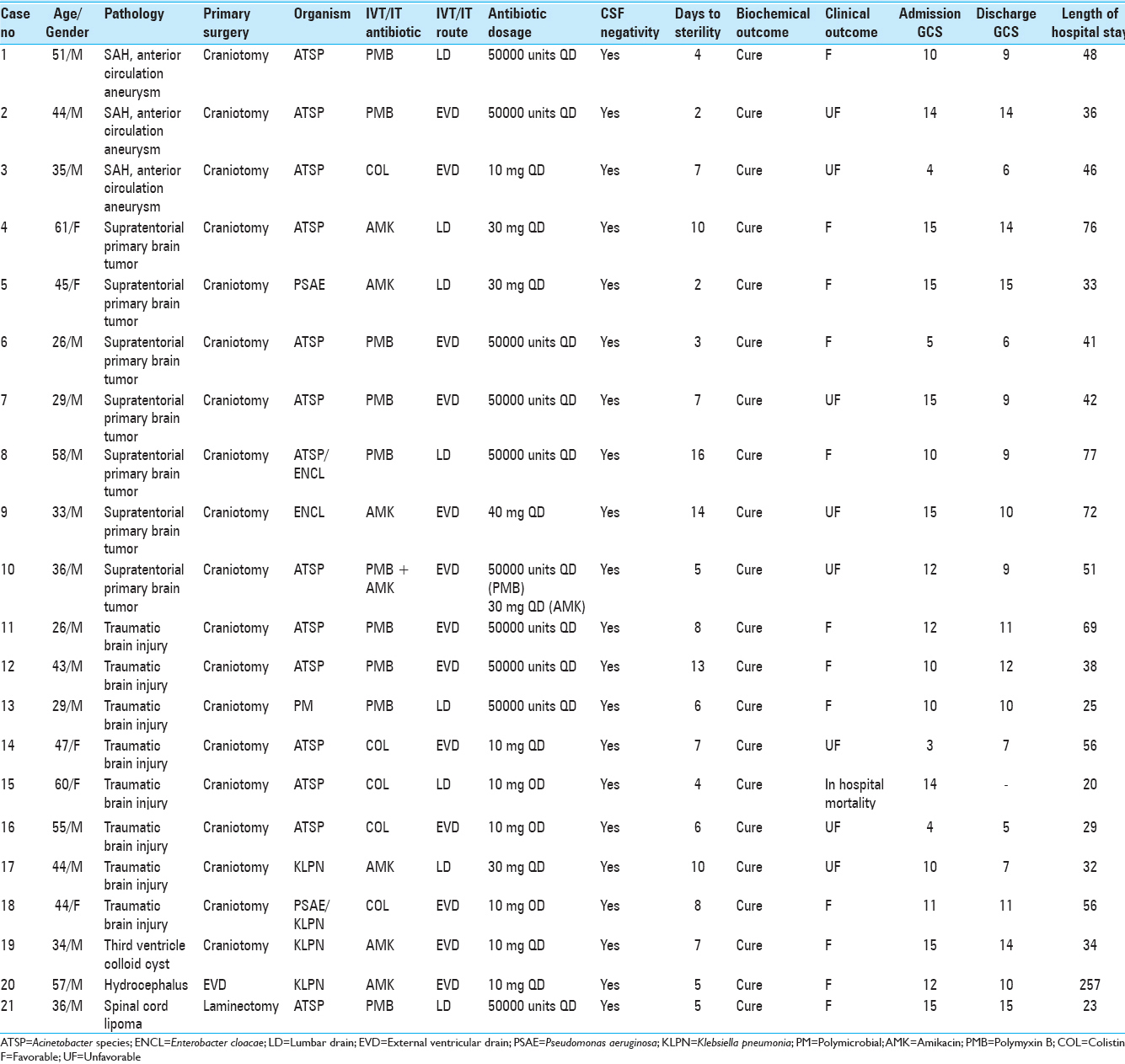
Out of the total 21 patients enrolled in the study, 16 were males and 5 were females. Their mean age was 41.7 ± 11 years (range: 26–58 years). All except one of these patients had cranial surgery. Details of individual cases are shown in
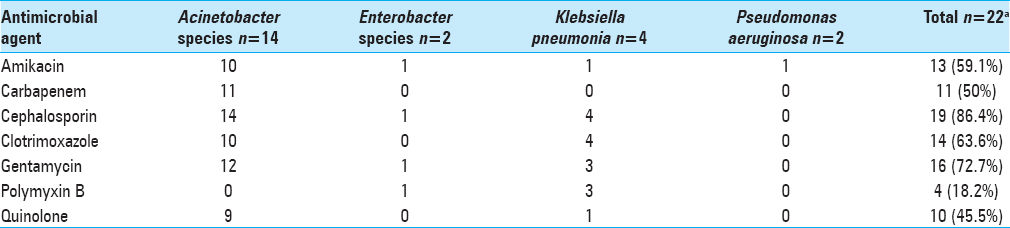
Acinetobacter species was the most common organism isolated on CSF culture (n = 14) followed by Klebsiella pneumoniae (n = 3), Pseudomonas aeruginosa (n = 1), and Enterobacter cloacae (n = 1). One patient had polymicrobial growth. Three antibiotics were used in the IVT/IT treatment group: Amikacin, polymyxin B, and colistin. The indication was primarily based on the sensitivity of pathogenic organisms cultured on CSF of individual patients;
Table 2
Antimicrobial resistance of causative organisms from CSF in 21 episodes of postneurosurgical gram-negative meningitis treated with IVT/IT therapy. Patient 8 and 18 had two causative organisms as shown in
Discharge GCS improved in 2, remained the same in 7, and deteriorated in 12 patients. Median hospital stay was 38 days (20–257 days), with the EVD group [46 (29–72)] having a longer stay than LD group [32.5 (20–76) days)]. At 6 months follow-up, favorable outcomes were seen in 14 (66.7%) patients and 7 (33.3%) patients remained in an unfavorable state. One (5%) patient died during treatment and although the patient had Acinetobacter species infection, the cause of death was found to be a massive pulmonary embolus. No adverse effects attributable to IVT/IT therapy were noted in the study.
DISCUSSION
Management of hospital acquired meningitis and/or ventriculitis is a daunting challenge for clinicians.[
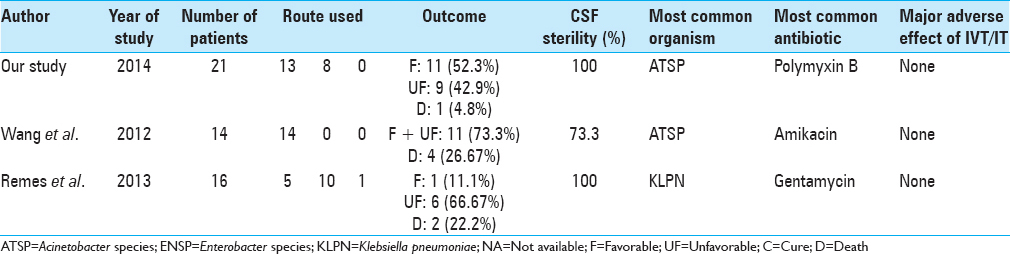
We herein report one of the largest series of patients with gram-negative CNS infections treated with IVT or IT therapy [
Earlier studies have debated the use of LD as the route for antibiotic administration as there remained speculations with regards to effective delivery of administered antibiotic into the ventricular cavity and CSF cistern. Kaiser et al. demonstrated that a 5–10 mg injection of antibiotic via lumbar route showed significantly lower ventricular CSF drug level when compared to lumbar CSF, while with ventricular dug injection the levels in the lumbar CSF were comparatively much higher.[
Postneurosurgical meningitis and/or ventriculitis have a critically high mortality rate, ranging from 3 to 33%.[
In our series, Acinetobacter species was the most commonly encountered gram-negative organism which makes this series differ from other reports, looking specifically at gram-negative meningitis/ventriculitis, where Pseudomonas species is usually more common.[
The major reason for the cautioned use of IVT/IT therapy has been the significant toxicity that was reported by earlier studies.[
CONCLUSION
The study describes the clinical outcomes of patients with gram-negative meningitis and/or ventriculitis being treated with IVT/IT antibiotics. In our study, there was good CSF sterility rate with no relapse. This, along with minimal side effects, makes IVT/IT an effective and relatively safe treatment option, especially in patients nonresponsive to standard intravenous therapy. However, prospective randomized trials with high sample size are needed to validate the findings.
Competing Interest
The authors declare that they have no financial or nonfinancial competing interest.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bhutia S, Srinivasan K, Ananthakrishnan N, Jayanthi S, Ravishankar M. Blood utilization in elective surgery—requirements, ordering and transfusion practices. Natl Med J India. 1996. 10: 164-8
2. Briggs S, Ellis-Pegler R, Raymond N, Thomas M, Wilkinson L. Gram-negative bacillary meningitis after cranial surgery or trauma in adults. Scand J Infect Dis. 2004. 36: 165-73
3. Caricato A, Pennisi M, Mancino A, Vigna G, Sandroni C, Arcangeli A. Levels of vancomycin in the cerebral interstitial fluid after severe head injury. Intensive Care Med. 2006. 32: 325-8
4. Clifford HE, Stewart GT. Intraventricular administration of a new derivative of polymyxin B in meningitis due to Ps. pyocyanea. Lancet. 1961. 2: 177-80
5. Falagas ME, Bliziotis IA, Tam VH. Intraventricular or intrathecal use of polymyxins in patients with Gram-negative meningitis: A systematic review of the available evidence. Int J Antimicrob Agents. 2007. 29: 9-25
6. Federico G, Tumbarello M, Spanu T, Rosell R, Iacoangeli M, Scerrati M. Risk factors and prognostic indicators of bacterial meningitis in a cohort of 3580 postneurosurgical patients. Scand J Infect Dis. 2001. 33: 533-7
7. Kaiser AB, McGee ZA. Aminoglycoside therapy of gram-negative bacillary meningitis. N Engl J Med. 1975. 293: 1215-20
8. Khawcharoenporn T, Apisarnthanarak A, Mundy LM. Intrathecal colistin for drug-resistant Acinetobacter baumannii central nervous system infection: A case series and systematic review. Clin Microbiol Infect. 2010. 16: 888-94
9. Kim BN, Peleg AY, Lodise TP, Lipman J, Li J, Nation R. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009. 9: 245-55
10. Krcmery V, Ondrusova A, Bucko L, Kalavsky E. Predictors of mortality in paediatric nosocomial meningitis: Results from 12 years national survey. Scand J Infect Dis. 2006. 38: 744-5
11. Lu CH, Chang WN, Chuang YC. Resistance to third-generation cephalosporins in adult gram-negative bacillary meningitis. Infection. 1999. 27: 208-11
12. Mancebo J, Domingo P, Blanch L, Coll P, Net A, Nolla J. Post-neurosurgical and spontaneous gram-negative bacillary meningitis in adults. Scand J Infect Dis. 1986. 18: 533-8
13. McCracken GH, Mize SG, Threlkeld N. Intraventricular gentamicin therapy in gram-negative bacillary meningitis of infancy. Report of the Second Neonatal Meningitis Cooperative Study Group. Lancet. 1980. 1: 787-91
14. Ng J, Gosbell IB, Kelly JA, Boyle MJ, Ferguson JK. Cure of multiresistant Acinetobacter baumannii central nervous system infections with intraventricular or intrathecal colistin: Case series and literature review. J Antimicrob Chemother. 2006. 58: 1078-81
15. O'Neill E, Humphreys H, Phillips J, Smyth EG. Third-generation cephalosporin resistance among Gram-negative bacilli causing meningitis in neurosurgical patients: Significant challenges in ensuring effective antibiotic therapy. J Antimicrob Chemother. 2006. 57: 356-9
16. Ondrusova A, Kalavsky E, Rudinsky B, Freybergh FP, Bauer F, Miklosko J. Pseudomonas aeruginosa causing nosocomial meningitis in neonates and children: Overview of 15 cases within 10 years. Neuro Endocrinol Lett. 2007. 28: 20-1
17. Parodi S, Lechner A, Osih R, Vespa P, Pegues D. Nosocomial enterobacter meningitis: Risk factors, management, and treatment outcomes. Clin Infect Dis. 2003. 37: 159-66
18. Remes F, Tomas R, Jindrak V, Vanis V, Setlik M. Intraventricular and lumbar intrathecal administration of antibiotics in postneurosurgical patients with meningitis and/or ventriculitis in a serious clinical state. J Neurosurg. 2013. 119: 1596-602
19. Rodriguez Guardado A, Blanco A, Asensi V, Perez F, Rial JC, Pintado V. Multidrug-resistant Acinetobacter meningitis in neurosurgical patients with intraventricular catheters: Assessment of different treatments. J Antimicrob Chemother. 2008. 61: 908-13
20. Roitberg BZ, Khan N, Alp MS, Hersonskey T, Charbel FT, Ausman JI. Bedside external ventricular drain placement for the treatment of acute hydrocephalus. Br J Neurosurg. 2001. 15: 324-7
21. Saleem AF, Ahmed I, Mir F, Ali SR, Zaidi AK. Pan-resistant Acinetobacter infection in neonates in Karachi, Pakistan. J Infect Dev Ctries. 2010. 4: 30-7
22. Talon D, Bailly P, Bertrand X, Thouverez M, Mulin B. Clinical and molecular epidemiology of chromosome-mediated resistance to third-generation cephalosporins in Enterobacter isolates in eastern France. Clin Microbiol Infect. 2000. 6: 376-84
23. Tangden T, Enblad P, Ullberg M, Sjolin J. Neurosurgical gram-negative bacillary ventriculitis and meningitis: A retrospective study evaluating the efficacy of intraventricular gentamicin therapy in 31 consecutive cases. Clin Infect Dis. 2011. 52: 1310-6
24. van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010. 362: 146-54
25. Wang JH, Lin PC, Chou CH, Ho CM, Lin KH, Tsai CT. Intraventricular antimicrobial therapy in postneurosurgical gram-negative bacillary meningitis or ventriculitis: A hospital-based retrospective study. J Microbiol Immunol Infect. 2014. 47: 204-10
26. Wang KW, Chang WN, Huang CR, Tsai NW, Tsui HW, Wang HC. Post-neurosurgical nosocomial bacterial meningitis in adults: Microbiology, clinical features, and outcomes. J Clin Neurosci. 2005. 12: 647-50
27. Wiesel J, Rose DN, Silver AL, Sacks HS, Bernstein RH. Lumbar puncture in asymptomatic late syphilis. An analysis of the benefits and risks. Arch Intern Med. 1985. 145: 465-8
28. Ziai WC, Lewin JJ. Improving the role of intraventricular antimicrobial agents in the management of meningitis. Curr Opin Neurol. 2009. 22: 277-82