Intratumoral hemorrhage in jugular foramen schwannoma after stereotactic radiosurgery: A case report
- Department of Neurosurgery, the University of Tokyo Hospital, Tokyo, Japan.
Correspondence Address:
Mariko Kawashima, Department of Neurosurgery, the University of Tokyo Hospital, Tokyo, Japan.
DOI:10.25259/SNI_550_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mariko Kawashima, Hirotaka Hasegawa, Masahiro Shin, Yuki Shinya, Nobuhito Saito. Intratumoral hemorrhage in jugular foramen schwannoma after stereotactic radiosurgery: A case report. 30-Sep-2021;12:479
How to cite this URL: Mariko Kawashima, Hirotaka Hasegawa, Masahiro Shin, Yuki Shinya, Nobuhito Saito. Intratumoral hemorrhage in jugular foramen schwannoma after stereotactic radiosurgery: A case report. 30-Sep-2021;12:479. Available from: https://surgicalneurologyint.com/surgicalint-articles/11149/
Abstract
Background: Clinically significant intratumoral hemorrhage is a rare complication of stereotactic radiosurgery (SRS) for benign tumors.
Case Description: Here, we present the case of a 64-year-old man who underwent SRS for a relatively large dumbbell-shaped left jugular foramen schwannoma (JFS) and thereafter developed intratumoral hemorrhage. On post-SRS day 3, he developed lower cranial nerve palsies with radiographically evident tumor expansion. His neurological conditions had gradually improved thereafter; however, he suddenly developed headache, dizziness, and mild hearing deterioration at 7 months due to intratumoral hemorrhage. We managed the patient conservatively, and eventually, his symptoms improved except for slight ataxia and hearing deterioration. Follow-up images at 4 years from SRS demonstrated significant tumor shrinkage. This is the first report describing intratumoral hemorrhage after SRS for JFS.
Conclusion: Transient expansion of the tumor and subsequent venous stasis around the tumor may have played a role in the hemorrhage. Intratumoral hemorrhage should be considered as a rare, but potential complication of SRS for JFSs.
Keywords: Intratumoral hemorrhage, Jugular foramen schwannoma, Radiation-induced adverse event, Stereotactic radiosurgery
INTRODUCTION
Jugular foramen schwannoma (JFS) is a rare intracranial tumor arising from cranial nerves IX, X, or XI, accounting for 2.9–4% of all intracranial schwannomas, which in turn accounts for 8% of all primary intracranial tumors.[
CASE PRESENTATION
A 64-year-old man without known significant medical history was referred to our hospital for the treatment of an incidentally found left JFS. He was found to be completely intact on examination and no family history of neurofibromatosis type 2. MRI revealed a dumbbell-shaped solid mass at the left cerebellopontine angle with marked enlargement of the affected jugular foramen and no sign of dural tail, which was 25 × 27 × 18 mm and 22 × 25 × 19 mm for the intradural and extradural portions, respectively, and 8.4 ml in total volume. The mass mildly compressed brainstem and had no evident sign of hemorrhage [
Figure 1:
(a-d) Pre-SRS images of the left jugular foramen schwannoma: (a) pre-SRS axial enhanced T1-weighted MRI used in radiosurgery; (b) pre-SRS coronal enhanced T1-weighted MRI used in radiosurgery; (c) MR venography showing the obstructed left jugular bulb; (d) positron emission tomography with fluorodeoxyglucose. These images revealed a well-enhanced, dumbbell-shaped, solid mass extending to the intracranial- and extracranial space, causing expansion of the jugular foramen and invading the hypoglossal canal. (e-i) Chronological changes on axial enhanced T1-weighted MRI at the level of the internal auditory canal where brainstem compression was evident, and computed tomography at the time of the intratumoral hemorrhage; (e) pre-SRS; (f) tumor expansion was observed 5 days after SRS; (g) further expansion 5 months after SRS; (h) computed tomography indicating intratumoral hemorrhage 7 months after SRS; (i) evident tumor shrinkage at the last follow-up 4 years after SRS. SRS: stereotactic radiosurgery, MR: Magnetic resonance, MRI: Magnetic resonance image.
Post-radiosurgical course
Three days after SRS, the patient developed dysphagia, hoarseness, and leftward deviation of the tongue that were confirmed by otorhinolaryngologic examinations, suggesting injuries of the left vagal and hypoglossal nerves. MRI on post-SRS day 5 showed tumor expansion without peritumoral edema [
Although his symptoms improved, follow-up MRI at 5 months from SRS showed further tumor expansion with central necrosis and peritumoral edema, albeit asymptomatic [
At 7 months, he suddenly developed headache, dizziness, mild hearing deterioration (20 dB decrease in pure tone audiometry), nausea, and vomiting. Imaging studies revealed intratumoral hemorrhage with exacerbation of peritumoral edema [
DISCUSSION
We experienced a JFS case which was accompanied with acute tumor expansion after SRS and subsequent clinically significant intratumoral hemorrhage. Intratumoral hemorrhage of intracranial schwannoma, particularly histologically detected microhemorrhage, has been recently considered to be more common than previously believed owing to advances in imaging studies and larger analyses.[
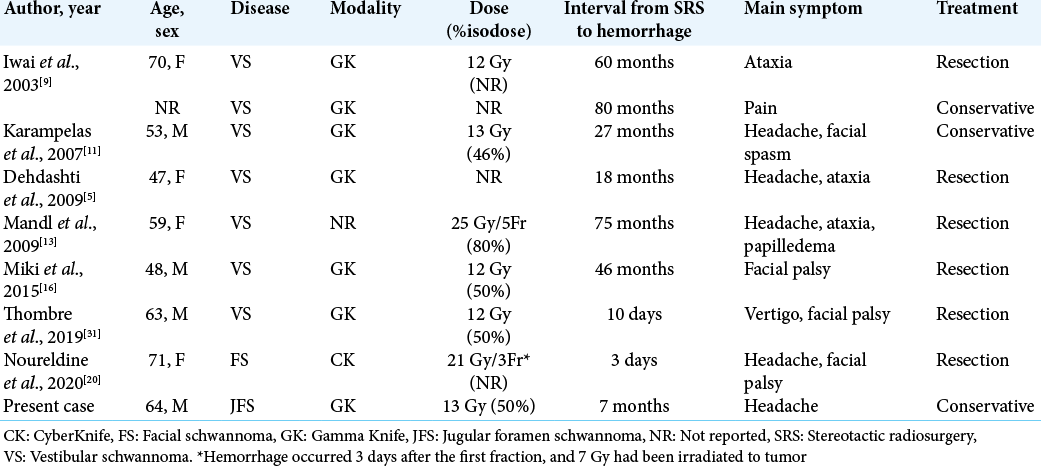
The influence of radiation on the intratumoral hemorrhage of intracranial schwannoma has not been fully elucidated. In particular, limited to clinically significant hemorrhage, there are eight reported cases in literature [
The optimal treatment for the intratumoral hemorrhage with brainstem compression is basically resection. The previously reported post-SRS cases were managed surgically in six cases and conservatively in two other cases, largely ending up in a good recovery [
CONCLUSION
This is the first case reported to demonstrate intratumoral hemorrhage after SRS for JFS. With conservative treatment, subsequent tumor shrinkage was observed in this case. While the etiology is not completely understood, radiation-induced tumor expansion and possible venous compromise are likely to cause the intratumoral hemorrhage. Although surgical resection is needed to be considered at first, conservative management could control the condition unless the hemorrhage is devastating or results in remarkable brainstem compression. Intratumoral hemorrhage should therefore be recognized as one of the potential sequelae after SRS for JFSs.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bulsara KR, Sameshima T, Friedman AH, Fukushima T. Microsurgical management of 53 jugular foramen schwannomas: Lessons learned incorporated into a modified grading system. J Neurosurg. 2008. 109: 794-803
2. Carlson ML, Tombers NM, Driscoll CL, van Gompel JJ, Lane JI, Raghunathan A. Clinically significant intratumoral hemorrhage in patients with vestibular schwannoma. Laryngoscope. 2017. 127: 1420-6
3. Cho YS, So YK, Park K, Baek CH, Jeong HS, Hong SH. Surgical outcomes of lateral approach for jugular foramen schwannoma: Postoperative facial nerve and lower cranial nerve functions. Neurosurg Rev. 2009. 32: 61-6
4. Choi CY, Soltys SG, Gibbs IC, Harsh GR, Sakamoto GT, Patel DA. Stereotactic radiosurgery of cranial nonvestibular schwannomas: Results of single-and multisession radiosurgery. Neurosurgery. 2011. 68: 1200-8
5. Dehdashti AR, Kiehl TR, Guha A. Vestibular schwannomas presenting with haemorrhage: Clinical presentation and histopathological evaluation of an unusual entity. Br J Neurosurg. 2009. 23: 431-6
6. Deibert CP, Ahluwalia MS, Sheehan JP, Link MJ, Hasegawa T, Yomo S. Bevacizumab for refractory adverse radiation effects after stereotactic radiosurgery. J Neurooncol. 2013. 115: 217-23
7. Elsharkawy M, Xu Z, Schlesinger D, Sheehan JP. Gamma knife surgery for nonvestibular schwannomas: Radiological and clinical outcomes. J Neurosurg. 2012. 116: 66-72
8. Hasegawa T, Kato T, Kida Y, Sasaki A, Iwai Y, Kondoh T. Gamma Knife surgery for patients with jugular foramen schwannomas: A multiinstitutional retrospective study in Japan. J Neurosurg. 2016. 125: 822-31
9. Iwai Y, Yamanaka K, Shiotani M, Uyama T. Radiosurgery for acoustic neuromas: Results of low-dose treatment. Neurosurgery. 2003. 53: 282-7
10. Kano H, Meola A, Yang HC, Guo WY, Martínez-Alvarez R, Martínez-Moreno N. Stereotactic radiosurgery for jugular foramen schwannomas: An international multicenter study. J Neurosurg. 2018. 129: 928-36
11. Karampelas I, Alberico RA, Plunkett RJ, Fenstermaker RA. Intratumoral hemorrhage after remote subtotal microsurgical resection and gamma knife radiosurgery for vestibular schwannoma. Acta Neurochir (Wien). 2007. 149: 313-6
12. Kim SH, Youm JY, Song SH, Kim Y, Song KS. Vestibular schwannoma with repeated intratumoral hemorrhage. Clin Neurol Neurosurg. 1998. 100: 68-74
13. Mandl ES, Vandertop WP, Meijer OW, Peerdeman SM. Imaging-documented repeated intratumoral hemorrhage in vestibular schwannoma: A case report. Acta Neurochir (Wien). 2009. 151: 1325-7
14. Martin JJ, Kondziolka D, Flickinger JC, Mathieu D, Niranjan A, Lunsford LD. Cranial nerve preservation and outcomes after stereotactic radiosurgery for jugular foramen schwannomas. Neurosurgery. 2007. 61: 76-81
15. Mathkour M, Helbig B, McCormack E, Amenta PS. Acute presentation of vestibular schwannoma secondary to intratumoral hemorrhage: A case report and literature review. World Neurosurg. 2019. 129: 157-63
16. Miki S, Ishikawa E, Yamamoto T, Akutsu H, Matsuda M, Sakamoto N. Extreme volume expansion of a vestibular schwannoma due to intratumoral hemorrhage after gamma knife radiosurgery. J Clin Neurosci. 2014. 22: 1196-9
17. Murakami K, Jokura H, Kawagishi J, Watanabe M, Tominaga T. Development of intratumoral cyst or extratumoral arachnoid cyst in intracranial schwannomas following gamma knife radiosurgery. Acta Neurochir (Wien). 2011. 153: 1201-9
18. Muthukumar N, Kondziolka D, Lunsford LD, Flickinger JC. Stereotactic radiosurgery for jugular foramen schwannomas. Surg Neurol. 1999. 52: 172-9
19. Niknafs YS, Wang AC, Than KD, Etame AB, Thompson BG, Sullivan SE. Hemorrhagic vestibular schwannoma: Review of the literature. World Neurosurg. 2014. 82: 751-6
20. Noureldine MH, Jha RT, Peto I, Malafronte PJ, Allen K, Agazzi S. Facial nerve schwannoma complicated by acute hemorrhage after treatment with stereotactic radiosurgery. World Neurosurg. 2020. 134: 128-32
21. Park CK, Kim DC, Park SH, Kim JE, Paek SH, Kim DG. Microhemorrhage, a possible mechanism for cyst formation in vestibular schwannomas. J Neurosurg. 2006. 105: 576-80
22. Peker S, Sengöz M, Kılıç T, Pamir MN. Gamma knife radiosurgery for jugular foramen schwannomas. Neurosurg Rev. 2012. 35: 549-53
23. Pollock BE, Foote RL, Stafford SL. Stereotactic radiosurgery: The preferred management for patients with nonvestibular schwannomas?. Int J Radiat Oncol Biol Phys. 2002. 52: 1002-7
24. Pollock BE, Kondziolka D, Flickinger JC, Maitz A, Lunsford LD. Preservation of cranial nerve function after radiosurgery for nonacoustic schwannomas. Neurosurgery. 1993. 33: 597-601
25. Samii M, Babu RP, Tatagiba M, Sepehrnia A. Surgical treatment of jugular foramen schwannomas. J Neurosurg. 1995. 82: 924-32
26. Sanna M, Bacciu A, Falcioni M, Taibah A. Surgical management of jugular foramen schwannomas with hearing and facial nerve function preservation: A series of 23 cases and review of the literature. Laryngoscope. 2006. 116: 2191-204
27. Sedney CL, Nonaka Y, Bulsara KR, Fukushima T. Microsurgical management of jugular foramen schwannomas. Neurosurgery. 2013. 72: 42-6
28. Sutiono AB, Kawase T, Tabuse M, Kitamura Y, Arifin MZ, Horiguchi T. Importance of preserved periosteum around jugular foramen neurinomas for functional outcome of lower cranial nerves: Anatomic and clinical studies. Neurosurgery. 2011. 69: ons230-40
29. Suzuki H, Toyoda S, Muramatsu M, Shimizu T, Kojima T, Taki W. Spontaneous haemorrhage into metastatic brain tumours after stereotactic radiosurgery using a linear accelerator. J Neurol Neurosurg Psychiatry. 2003. 74: 908-12
30. Tan LC, Bordi L, Symon L, Cheesman AD. Jugular foramen neuromas: A review of 14 cases. Surg Neurol. 1990. 34: 205-11
31. Thombre B, Sadashiva N, Krishnan JB, Prabhuraj AR, Rao KN, Arima A. Symptomatic post-radiosurgery intratumoral hemorrhage in a case of vestibular schwannoma: A case report and review of the literature. Stereotact Funct Neurosurg. 2019. 97: 399-403
32. Woo PY, Lam PL, Ip YH, Chan TS, Ng OK, Kwan MC. “When the benign bleed” vestibular schwannomas with clinically significant intratumoral hemorrhage: A case series and review of the literature. Asian J Neurosurg. 2021. 16: 221-7
33. Yang X, Liu J, Zhang Y, Richard SA. Tumor-associated hemorrhage in patients with vestibular schwannoma. Acta Neurochir (Wien). 2018. 160: 1563-9
34. Yates CW, Weinberg M, Packer MJ, Jacob A. Fatal case of tumor-associated hemorrhage in a large vestibular schwannoma. Ann Otol Rhinol Laryngol. 2010. 119: 402-5
35. Zhang N, Pan L, Dai JZ, Wang BJ, Wang EM, Cai PW. Gamma knife radiosurgery for jugular foramen schwannomas. J Neurosurg. 2002. 97: 456-8
36. Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: Mechanism, efficacy and issues. Mol Cancer. 2019. 18: 21