- Departments of Neurosurgery, Northwestern Feinberg School of Medicine, 420 East Superior Street, Chicago, Illinois, United States.
- Departments of Pathology, Northwestern Feinberg School of Medicine, 420 East Superior Street, Chicago, Illinois, United States.
- Department of Neurosurgery, University of California, Davis, California, United States.
Correspondence Address:
Constantine Karras
Department of Neurosurgery, University of California, Davis, California, United States.
DOI:10.25259/SNI_363_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ketan Yerneni, Constantine Karras, Hannah K. Weiss, Craig M. Horbinski, Orin Bloch. Intraventricular adult Taenia solium causing hydrocephalus: A case report. 18-Jul-2020;11:202
How to cite this URL: Ketan Yerneni, Constantine Karras, Hannah K. Weiss, Craig M. Horbinski, Orin Bloch. Intraventricular adult Taenia solium causing hydrocephalus: A case report. 18-Jul-2020;11:202. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10146
Abstract
Background: Neurocysticercosis (NCC) is the most common parasitic infection of the central nervous system worldwide and is caused by the larval form of the tapeworm Taenia solium. In general, T. solium larval form may be located in the neuraxis, resulting in pathology. Here, we report a rare case of female with a history of adult onset seizures presenting with adult form T. solium in the fourth ventricle, causing hydrocephalus.
Case Description: A 36-year-old female patient with a known history of adult onset seizures presented with a 1-year history of progressively worsening bilateral headaches with vertigo and intermittent nausea. A computerized tomography scan revealed ventriculomegaly and transependymal flow, with an obstruction at the level of the fourth ventricle. Outpatient magnetic resonance imaging demonstrated obstructive hydrocephalus secondary to a lobulated cystic mass within the fourth ventricle, demonstrating a gross appearance consistent with racemose NCC. The patient underwent endoscopic third ventriculostomy, and gross examination of the resected cyst revealed a mature T. solium larvae encased in a cystic membrane. Given that our patient was born and raised in Mexico but had not returned since the age of 8, NCC was an unexpected finding.
Conclusion: The present case highlights the importance of maintaining high suspicion for NCC in all patients presenting with seizures or hydrocephalus of unknown cause. Even in patients with a very remote history of residence in an endemic country, NCC can be an overlooked, underlying cause of both chronic neurologic symptoms, as well as acute, life-threatening neurologic emergencies.
Keywords: Hydrocephalus, Intraventricular, Neurocysticercosis, Taenia solium, Tapeworm
INTRODUCTION
Neurocysticercosis (NCC) is a parasitic disease endemic to most of the developing world and is a major source of admissions to neurological wards, as it is a significant cause of acquired seizures and epilepsy worldwide.[
CASE REPORT
History
Our patient is a 36-year-old female with a history of adult onset seizures. She was born and raised in Mexico and emigrated to the United States at the age of 8. Since arriving to the United States, she had never returned to Mexico. She had her first seizure about 10 years before presentation, at which point she was placed on anti-epileptic medications that were subsequently weaned off without any further seizure activity in the past several years. No prior imaging was available for our review, but the patient denied report of any intracranial lesions that could be identified as the culprit of the adult onset seizures.
Examination
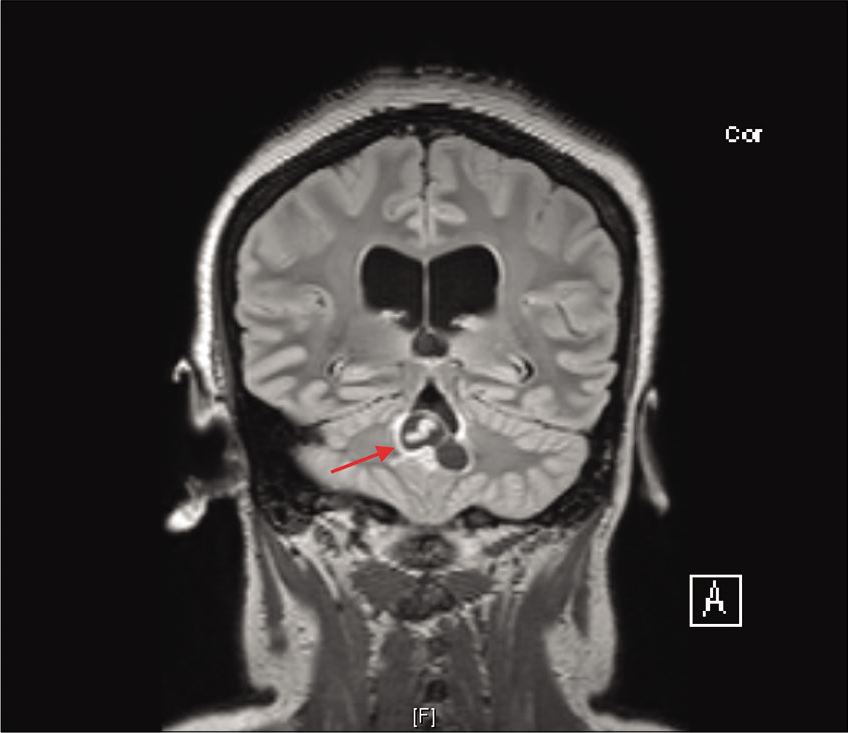
She initially presented to an outside institution with progressively worsening bilateral headaches over the past year that were associated with vertigo and intermittent nausea. The headaches were worse at night and on waking up in the morning, and they improved throughout the day. A computerized tomography (CT) scan demonstrated ventriculomegaly and transependymal flow, with evidence of obstruction at the level of the fourth ventricle. She was referred to our clinic by our neurology colleagues, where she was found to have no neurologic deficits on examination. Outpatient magnetic resonance imaging (MRI) demonstrated obstructed hydrocephalus secondary to a lobulated cystic mass within the fourth ventricle, with a streak of internal linear enhancement most consistent with racemose NCC. A worm was easily identified within the cyst on the MRI [
Operation
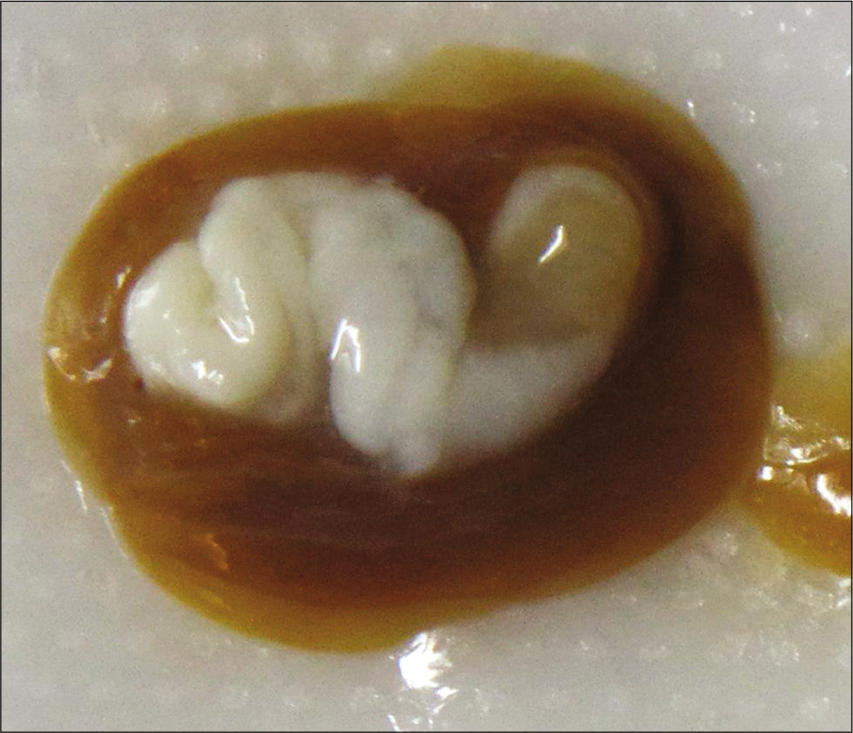
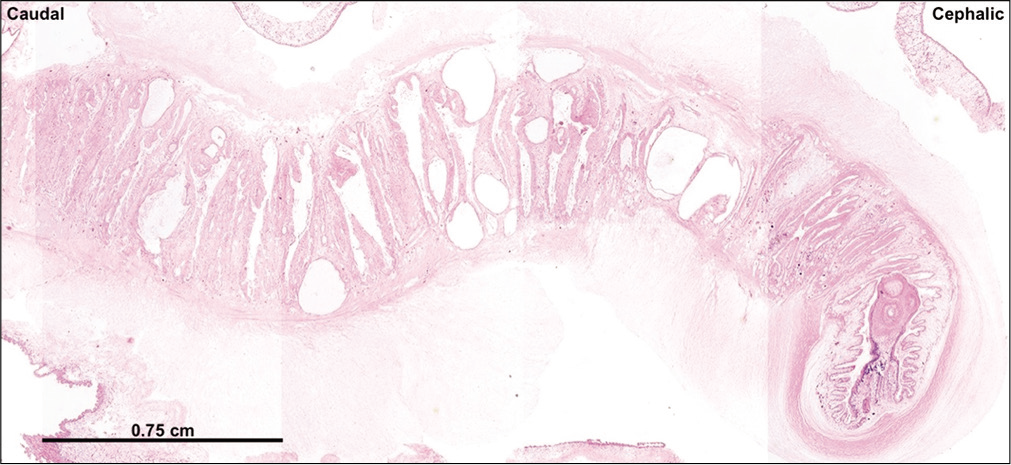
Given the chronic nature of the lesion and her benign clinical examination, she was scheduled for elective surgery a few weeks later. She underwent right frontal burr hole for endoscopic third ventriculostomy and placement of external ventricular drain (EVD), then she was positioned prone and underwent suboccipital craniotomy for resection of the fourth ventricular cystic mass followed by duraplasty. The cyst was removed as a single en bloc specimen, although during the process of removal, a small portion of the cyst ruptured into the ventricle – this was quickly suctioned and extensively irrigated. Gross examination [
Postoperative course
Postoperatively, she had no neurologic deficits. Her EVD was quickly weaned with minimal drainage and low intracranial pressure, so it was removed on postoperative day 2. She underwent ophthalmologic examination, demonstrating no evidence of an active ocular infection or involvement of NCC, but concern for a chronic, inactive choroidal inflammatory process. Our infectious disease colleagues were consulted and found no other risk factors for NCC aside from living in Mexico until the age of 8, and she was negative for tuberculosis infection. Given her longstanding history of seizures, but recent presentation with an active lesion, it was felt that she may have been auto-infecting. She underwent a 21-day course of albendazole with a 14-day dexamethasone taper for protection against aseptic meningitis. She was also prescribed a single dose of praziquantel at the end of her therapy given concerns for possible auto-infection from her gastrointestinal system. On discharge, her headaches, vertigo, and nausea had completely resolved.
DISCUSSION
NCC is the most common helminthic infection of the CNS worldwide and has recently become an emerging infectious disease in the United States, particularly in areas of high rates of immigration from endemic regions of Latin America.[
NCC has been widely reported in the literature; diagnostic criteria,[
Intraventricular NCC presents more aggressively than the parenchymal form, often causing intracranial hypertension due to CSF obstruction and potential arachnoiditis.[
The management and treatment of intraventricular NCC is distinct from the parenchymal form, which is the most widely published on. Indications for surgical management of intraventricular NCC include mass effect, CSF obstruction, and ventricular cysts. Acute hydrocephalus, as manifested in the case presented here, requires emergency ventriculostomy followed by cyst resection.[
In several cases, a ventriculostomy by itself may be enough to relieve hydrocephalus without a shunt; however, external drainage is often indicated in patients with extensive disease and damage. Furthermore, ventriculitis often complicates shunt placement in patients with NCC, resulting in shunt malfunction rates that are exceptionally high – reported to be over 30% – with a high rate of postoperative mortality.[
At present, there are no clinical trials or definitively established guidelines for the anthelminthic treatment of intraventricular NCC and remain poorly resolved; lesions may spontaneously resolve, and anthelminthic agents may lead to worsening of symptoms.[
The present case highlights the importance of maintaining high suspicion for NCC in all patients presenting with seizures or hydrocephalus of unknown cause. Patients are most effectively evaluated for NCC by obtaining an MRI, serologic testing, and thorough neurologic examination. In addition, in the era of globalization and increased immigration and travel, it is critical to collect a thorough social history, including travel and residence history to any endemic country. This case illustrates that even in patients with a very remote history of residence in an endemic country, NCC can be an overlooked, underlying cause of both chronic neurologic symptoms, as well as acute, life- threatening neurologic emergencies.
CONCLUSION
Although rare in the United States, increased travel and immigration across endemic areas have increased the incidence of NCC in regions unfamiliar with the disease. Intraventricular cysticerci may be mistaken for other common intraventricular lesions, including colloid cysts. Here, we have presented an extremely rare case of an adult T. Solium within a cyst in the fourth ventricle, causing acute hydrocephalus.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abba K, Ramaratnam S, Ranganathan LN. Anthelmintics for people with neurocysticercosis. Cochrane Database Syst Rev. 2010. 20: Cd000215-
2. Amaral L, Maschietto M, Maschietto R, Cury R, Ferreira NF, Mendonça R. Ununsual manifestations of neurocysticercosis in MR imaging: Analysis of 172 cases. Arq Neuropsiquiatr. 2003. 61: 533-41
3. Baird RA, Wiebe S, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: Treatment of parenchymal neurocysticercosis: Report of the guideline development subcommittee of the American academy of neurology. Neurology. 2013. 80: 1424-9
4. DeGiorgio CM, Houston I, Oviedo S, Sorvillo F. Deaths associated with cysticercosis. Report of three cases and review of the literature. Neurosurg Focus. 2002. 12: e2-
5. Del Brutto OH. Neurocysticercosis: A review. Sci World J. 2012. 2012: 159821-
6. Del Brutto OH, Rajshekhar V, White AC, Tsang VC, Nash TE, Takayanagui OM. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001. 57: 177-83
7. Figueroa JJ, Davis LE, Magalhaes A. Extraparenchymal neurocysticercosis in Albuquerque, New Mexico. J Neuroimaging. 2011. 21: 38-43
8. Jensen TO, Post JJ. Intraventricular neurocysticercosis: Presentation, diagnosis and management. Asian Pac J Trop Med. 2016. 9: 815-8
9. Kelley R, Duong DH, Locke GE. Characteristics of ventricular shunt malfunctions among patients with neurocysticercosis. Neurosurgery. 2002. 50: 757-61
10. Kraft R. Cysticercosis: An emerging parasitic disease. Am Fam Physician. 2007. 76: 91-6
11. Proaño-Narvaez JV, Meza-Lucas A, Mata-Ruiz O, García-Jerónimo RC, Correa D. Laboratory diagnosis of human neurocysticercosis: Double-blind comparison of enzyme-linked immunosorbent assay and electroimmunotransfer blot assay. J Clin Microbiol. 2002. 40: 2115-8
12. Rajshekhar V. Surgical management of neurocysticercosis. Int J Surg. 2010. 8: 100-4
13. Rangel-Castilla L, Serpa JA, Gopinath SP, Graviss EA, Diaz-Marchan P, White AC Jr. Contemporary neurosurgical approaches to neurocysticercosis. Am J Trop Med Hyg. 2009. 80: 373-8
14. Serpa JA, Graviss EA, Kass JS, White AC Jr. Neurocysticercosis in Houston, Texas: An update. Medicine (Baltimore). 2011. 90: 81-6
15. Shahani L, Garnes ND, Mejia R. Intraventricular Taenia solium cysts presenting with Bruns syndrome and indications for emergent neurosurgery. Am J Trop Med Hyg. 2015. 92: 1261-4
16. Sharma BS, Sawarkar DP, Verma SK. Endoscopic management of fourth ventricle neurocysticercosis: Description of the new technique in a case series of 5 cases and review of the literature. World Neurosurg. 2019. 122: e647-54
17. Sinha S, Sharma BS. Intraventricular neurocysticercosis: A review of current status and management issues. Br J Neurosurg. 2012. 26: 305-9
18. Suri A, Goel RK, Ahmad FU, Vellimana AK, Sharma BS, Mahapatra AK. Transventricular, transaqueductal scope-in-scope endoscopic excision of fourth ventricular neurocysticercosis: A series of 13 cases and a review. J Neurosurg Pediatr. 2008. 1: 35-9
19. White AC Jr. Neurocysticercosis: Updates on epidemiology, pathogenesis, diagnosis, and management. Annu Rev Med. 2000. 51: 187-206
20. White AC, Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the infectious diseases society of America (IDSA) and the American society of tropical medicine and hygiene (ASTMH). Am J Trop Med Hyg. 2018. 98: 945-66