- Department of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan
- Department of Radiology, Aga Khan University Hospital, Karachi, Pakistan
Correspondence Address:
Zanib Javed, Department of Neurosurgery, Aga Khan University Hospital, Aga Khan University Hospital, Karachi, Pakistan.
DOI:10.25259/SNI_224_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Zanib Javed1, Zunaira Saeed1, Sibgha Khan2, Altaf Ali Laghari1. Invasive pituitary adenoma presenting with cerebrospinal fluid rhinorrhea and meningitis – A case report. 06-Sep-2024;15:318
How to cite this URL: Zanib Javed1, Zunaira Saeed1, Sibgha Khan2, Altaf Ali Laghari1. Invasive pituitary adenoma presenting with cerebrospinal fluid rhinorrhea and meningitis – A case report. 06-Sep-2024;15:318. Available from: https://surgicalneurologyint.com/surgicalint-articles/13084/
Abstract
Background: Most pituitary neuroendocrine tumors are benign, except some adenomas that show invasiveness and are called invasive pituitary adenomas. These are challenging and rare pathologies.
Case Description: We present a case of a 40-year-old male who presented to the emergency with seizures, rhinorrhea, headache, and drowsiness. Radiology images showed a sellar mass with supra-sellar extension and pneumocephalus. The pituitary profile was within normal limits. The patient underwent bifrontal craniotomy and maximum safe resection of the lesion with cerebrospinal fluid (CSF) leak repair and lumbar drain insertion. Histological examination and immunohistochemical stain were consistent with pituitary adenoma. Postoperatively, there was no CSF leak, and the patient’s Glasgow Coma Scale improved.
Conclusion: Rhinorrhea is a unique presentation for pituitary adenoma. According to the current literature, surgery is the only effective treatment as part of the management of invasive pituitary adenomas, along with a multidisciplinary approach.
Keywords: Invasive pituitary adenomas, Meningitis, Pneumocephalus, Rhinorrhea
INTRODUCTION
Around 10–15% of all intracranial tumors will be pituitary adenomas, 80% of which will be benign pituitary adenomas, and the rest will be substantial risk adenomas or metastatic tumors.[
CASE PRESENTATION
A 40-year-old gentleman sought urgent medical attention at the emergency department, reporting a 2-year history of untreated seizures. He presented with a sudden onset of watery discharge from his nose, accompanied by a persistent headache and drowsiness over the past day. Upon arrival, his vital signs were stable, and a thorough general examination revealed no significant abnormalities. However, neurological evaluation disclosed concerning findings, including a Glasgow Coma Scale (GCS) score of E1V1M5, along with evident cerebrospinal fluid (CSF) leak and bilaterally reactive pupils. A computed tomography scan was done in the emergency department, which showed a significantly distended sella housing low attenuation mass lesion measuring 35 × 27 mm (about 1.06 in), causing significant thinning of the adjacent sella bone with erosion. On the left side of the suprasellar region, there was a soft tissue mass measuring 22 × 13 mm (about 0.51 in) with adjacent mild perilesional edema, an extension of the sellar lesion, or tumor bleed. Multiple small, scattered foci of air specks were noted, most of which were in the subarachnoid space representing pneumocephalus [
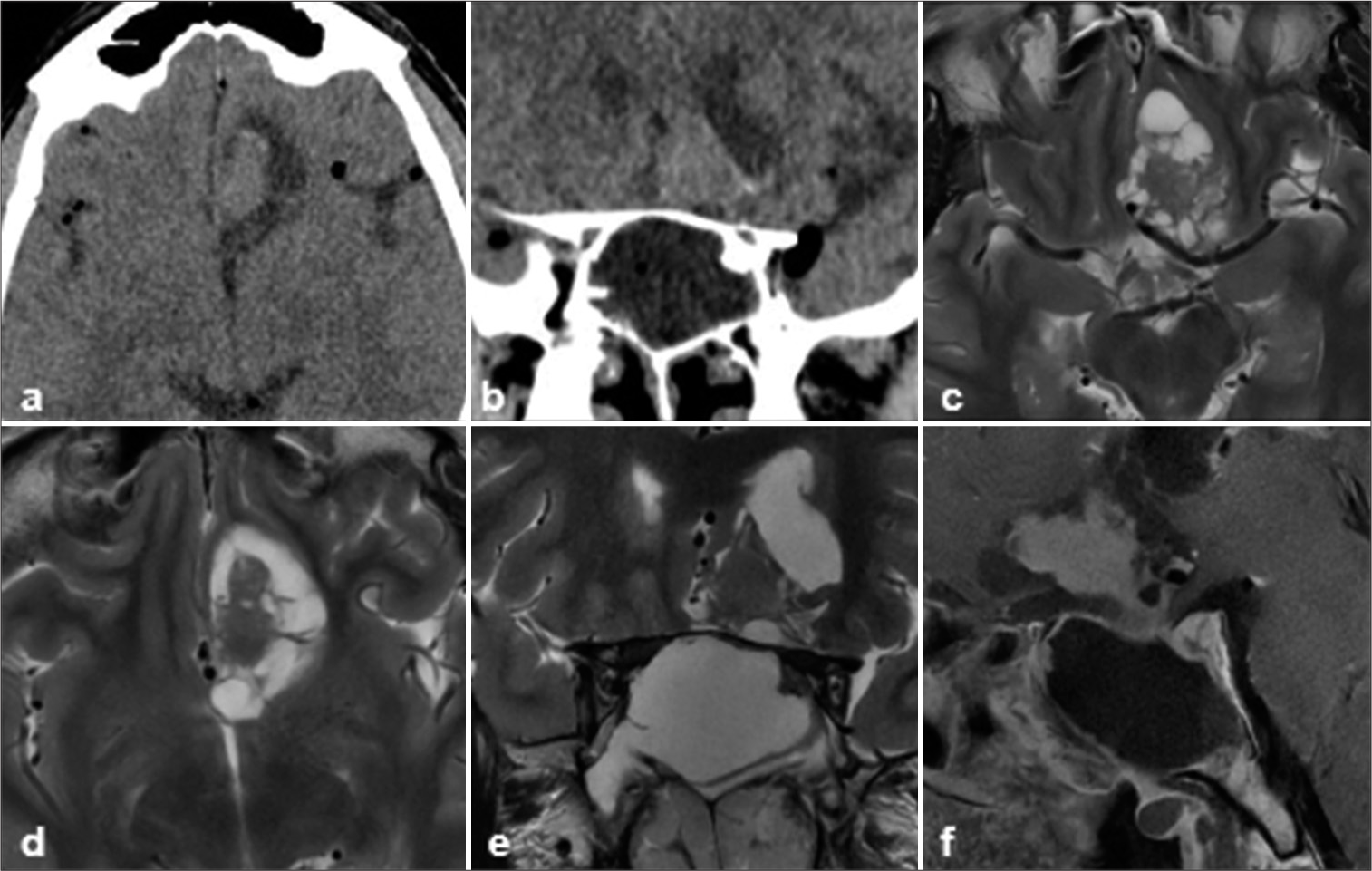
Figure 1:
(a and b) Computed tomography scan axial sections showing the sella with pneumocephalus and lesion in suprasellar location. (c and d) axial T2-weighted images showing lobulated solid cum cystic lesion in the left suprasellar location, abutting the frontal lobe parenchyma and anterior cerebral arteries, (e) coronal T2-weighted image showing the same lesion in suprasellar location with expanded sella. (f) Sagittal T1 postcontrast image showing expanded sella with a lesion in suprasellar location and along posterior wall. The solid part of the lesion is showing homogenous enhancement.
The patient was intubated in the emergency department due to low GCS and managed in the intensive care unit. Broad-spectrum IV antibiotics were started. The pituitary profile showed normal levels of thyroid-stimulating hormone and free thyroxine with slightly low free triiodothyronine. Follicle-stimulating hormone, luteinizing hormone, prolactin, testosterone, cortisol, and insulin-like growth factor-1 were within normal limits. Brain magnetic resonance imaging (MRI) demonstrated an expansile solid cum cystic lesion measuring 35 × 35 × 25 mm (about 0.98 in) in anteroposterior × transverse × craniocaudal in the sella with predominant cystic component and peripheral enhancing nodular soft-tissue rim dimensions. There was an obliteration of diaphragmatic sella with intracranial extension of the lesion in the left parasagittal location with mild surrounding edema. This part of the lesion showed postcontrast enhancement and restriction on diffusion-weighted images. Foci of signal dropout were noted on susceptibility-weighted imaging representing hemorrhage measuring 25 × 15.5 mm in axial dimensions. Abnormal patchy intra-ventricular enhancement was seen in the posterior horns of lateral ventricles. The cystic component was laterally abutting the internal carotid and cavernous sinus bilaterally. Anteriorly, it was reaching up to the cribriform plate. Posteriorly, it was abutting the pituitary gland; however, it was separately visualized and was being pushed by the lesion. The pituitary stalk was deviated to the right side. There was a diffuse meningeal enhancement. Anteriorly, the lesion reached up to the pterygopalatine fossa bilaterally (more on the right side) with erosion of the skull base anterior to the lacerum [
The patient underwent a neuro-navigation guided bi-frontal craniotomy for the maximal safe resection of the lesion and repair of the cerebrospinal fluid (CSF) leak. The lesion was identified as a cystic and solid component within the sella, extended to the point of causing erosion of the sphenoid bone. A 5 mL sample of necrotic tumor, resembling thick purulent liquid, was aspirated and sent for microbiological culture. The tumor was then gradually debulked by piecemeal removal. Reconstruction and repair of the CSF leak involved covering the cribriform plate and sellar floor with a fascia lata graft, followed by the application of fat, surgical sealant, and Spongostan. Subsequent analysis of a frozen section of the tumor indicated a single piece of tissue measuring 0.5 × 0.4 cm, labeled as a neoplastic sellar lesion, with the potential for metastasis or lymphoma not entirely ruled out. The lumbar drain was kept for 5 days. Histopathological examination revealed fragments of a neoplastic lesion composed of sheets and nests of cells having round-to-oval hyperchromatic nuclei with stippled chromatin and moderate pale cytoplasm. Immunohistochemical stains were performed, which showed the following reactivity pattern: synaptophysin positive, CKAE1/AE3 negative, CD20 negative, CD3 negative, GFAP negative, and Ki-67 low. There was no evidence of malignancy, thereby confirming the diagnosis of pituitary adenoma based on morphological features and immunohistochemical profile [
Figure 2:
Histopathology. (a) Haematoxylin and eosin stain sections reveal fragments of neoplastic cells composed of sheets and nests of cells having round to oval hyperchromatic nuclei with stippled chromatin and moderate pale cytoplasm. (b) Immunohistochemical stains show low MIB, (c) CKAE1/AE3 negative and (d) Synaptophysin positive.
Postoperatively, the patient was managed in the intensive care unit with infectious disease and endocrinology on board. He developed partial left third nerve palsy. There was no CSF leak or seizures. Due to difficulty in weaning, a tracheostomy was done on the 6th postoperative day. The patient’s GCS improved to E4VTM6. Subsequent CSF cultures showed no growth of any organism. The lumbar drain was removed on the 5th postoperative day, and there were no complications. The patient was discharged on the 15th postoperative day. Postoperative MRI after 3 months showed some residual disease. He received stereotactic radiosurgery of 25 Gy in 5 fractions after 6 months of surgery. At his last follow-up 1 year after surgery, the patient’s progress was encouraging, with stable disease progression noted [
Figure 3:
(a-c) Axial and sagittal T2-weighted images resection of previously seen solid cum cystic lesion in the left suprasellar location with postsurgical changes. (d-f) T1 postcontrast images axial, sagittal, and coronal showing small enhancing residual lesions in the suprasellar location. The residual sellar lesion shows homogenous enhancement.
CASE DISCUSSION
About 5% of pituitary adenomas become locally invasive. Rapid progression of symptoms and invasiveness is the defining criterion that differentiates locally invasive pituitary adenomas from benign adenomas. The genetic make-up of these tumors may differ from more benign adenomas, even though the histology is similar.[
In the literature, most instances involving CSF leaks (73%) happened after the start of medical treatment, but in about 27% of the cases, the CSF leak emerged as a presenting symptom of a pituitary adenoma. 81% of patients who developed CSF leaks following initiation of medical therapy had prolactinomas. 11% had nonfunctioning pituitary adenoma, 4% had a GH-secreting adenoma, 2% had mammosomatotroph cell adenoma, and 2% had adrenocorticotrophic hormone (ACTH)-secreting adenoma.[
Patients who developed spontaneous (noniatrogenic) CSF leaks, like our patient, had a variety of pituitary adenomas, with 42% having prolactinomas, 42% nonfunctioning pituitary adenomas (as in the case of our patient), 8% GH-secreting adenomas, and 8% ACTH-secreting adenomas.[
Wilson’s system (modified from Hardy) of anatomic classification of pituitary adenoma grades these tumors according to the extent of suprasellar and parasellar extension as well as invasion into the floor of sella, sphenoid bone, and distant spread.[
A review of treatment modalities showed surgical intervention as the definitive treatment for the CSF leak carried out in 88% of cases. The most common definitive procedures for CSF leaks included trans-sphenoidal surgery (70%). Craniotomy was done in 11% (also employed in the case of our patient), and lumboperitoneal shunt in 4%.[
CONCLUSION
Locally invasive pituitary adenomas, impacting 5% of cases, show rapid progression and often cause optic compression and CSF leaks. Our patient, with a nonfunctioning pituitary adenoma, had an intracranial and extracranial extension and CSF leak without hydrocephalus. Surgery with tumor resection and skull base reconstruction is the main treatment for CSF leaks, with dopamine agonist withdrawal and bed rest as adjuncts in cases of prolactinomas. Recurrence, though rare, may require further surgery. Notably, our patient showed sustained improvement without CSF leak recurrence, even 1-year postsurgery, indicating successful management.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Amar AP, Hinton DR, Krieger MD, Weiss MH. Invasive pituitary adenomas: Significance of proliferation parameters. Pituitary. 1999. 2: 117-22
2. Boscolo M, Baleriaux D, Bakoto N, Corvilain B, Devuyst F. Acute aseptic meningitis as the initial presentation of a macroprolactinoma. BMC Res Notes. 2014. 7: 9
3. Cohn EM, editors. Handbook of neurosurgery. Neuroophthalmology. 2011. 35: 54
4. Esposito D, Olsson DS, Ragnarsson O, Buchfelder M, Skoglund T, Johannsson G. Non-functioning pituitary adenomas: Indications for pituitary surgery and post-surgical management. Pituitary. 2019. 22: 422-34
5. Glezer A, Bronstein MD. Prolactinoma. Arq Bras Endocrinol Metabol. 2014. 58: 118-23
6. Kanemitsu T, Ikeda N, Fukumura M, Sakai S, Oku H, Furuse M. Pituitary stone resulting in visual dysfunction and spontaneous rhinorrhea in nonfunctioning pituitary adenoma: Illustrative case. J Neurosurg Case Lessons. 2021. 1: CASE2029
7. Lam G, Mehta V, Zada G. Spontaneous and medically induced cerebrospinal fluid leakage in the setting of pituitary adenomas: Review of the literature. Neurosurg Focus. 2012. 32: E2
8. Møller MW, Andersen MS, Glintborg D, Pedersen CB, Halle B, Kristensen BW. Pituitary adenoma. Ugeskr Laeger. 2019. 181: V05180331
9. Sav A, Rotondo F, Syro LV, Di Ieva A, Cusimano MD, Kovacs K. Invasive, atypical and aggressive pituitary adenomas and carcinomas. Endocrinol Metab Clin North Am. 2015. 44: 99-104
10. Serioli S, Doglietto F, Fiorindi A, Biroli A, Mattavelli D, Buffoli B. Pituitary adenomas and invasiveness from anatomo-surgical, radiological, and histological perspectives: A systematic literature review. Cancers (Basel). 2019. 11: 1936
11. Vieira Neto L, Boguszewski CL, Araújo LA, Bronstein MD, Miranda PA, Musolino NR. A review on the diagnosis and treatment of patients with clinically nonfunctioning pituitary adenoma by the neuroendocrinology department of the Brazilian society of endocrinology and metabolism. Arch Endocrinol Metab. 2016. 60: 374-90