- Department of Neurosurgery, Military-Medical Academy S. M. Kirov, Saint-Petersburg, Russian Federation.
Correspondence Address:
Artem Rafaelyan, Department of Neurosurgery, Military-Medical Academy S. M. Kirov, Saint-Petersburg, Russian Federation.
DOI:10.25259/SNI_533_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Artem Rafaelyan, Dmitry V. Svistov. Isolated hypoglossal nerve neuropathy in vertebral dolichoectasia: Microvascular decompression by vessel transposition with Teflon cuff. 05-Aug-2022;13:336
How to cite this URL: Artem Rafaelyan, Dmitry V. Svistov. Isolated hypoglossal nerve neuropathy in vertebral dolichoectasia: Microvascular decompression by vessel transposition with Teflon cuff. 05-Aug-2022;13:336. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11759
Abstract
Background: A clinical case of isolated unilateral hypoglossal nerve (HN) neuropathy, which spontaneously occurred from vertebral artery dolichoectasia and was cured by a new method of microvascular decompression by transposition of the vertebral artery using the Teflon cuff.
Case Description: A young patient with an anamnesis of the disease for more than 4 years and complaints of a deviation of the tongue to the right and dysarthria was examined. MRI of the brain revealed compression of the medulla oblongata by an elongated, dilated, and deformed right vertebral artery. Compression of the medulla oblongata and HN was confirmed during surgery. A transposition of the vertebral artery was performed using a Teflon cuff in the ventral direction to the clivus. Three months after surgery, positive dynamics was noted in the form of regression of dysarthria and improvement of mobility and trophic language.
Conclusion: Thus, isolated HN neuropathy as a result of compression by an elongated, dilated, and deformed vertebral artery is a rare neurological disease that can be successfully treated by transposition using a Teflon cuff.
Keywords: Hypoglossal nerve, Microvascular decompression, Neuropathy, Transposition, Vertebral dolichoectasia
INTRODUCTION
The hypoglossal nerve (HN) provides motor innervation to the muscles of the tongue, as well as the genioglossus, styloglossus, and hypoglossus muscles. The nucleus of the HN is located in the lower parts of the medulla oblongata, near the floor of the fourth ventricle. Axons run ventrally to the anterolateral sulcus between the pyramid and the inferior olive and exit as part of a group of roots, connecting into 2 (80%) or 3 trunks (20%).[
Nerve damage disrupts the balancing action of the genioglossus muscle, which deviates the tongue in the opposite direction. Therefore, the neuropathy of the HN (NHN) leads to the classic triad of chewing difficulties, dysphagia, and dysarthria.
HN has five segments: the medullary, cisternal, skull bases, carotid space, and sublingual segment.[
NHN is a rare disease[
In the cisternal segment, HN damage can be caused by contact with the V4 segment of the vertebral artery,[
Tumors are the most common cause of NHN and can affect every segment of the nerve, but most commonly the one passing through the canal of the HN at the base of the skull. In the segment of the skull base, NHN can occur due to craniocerebral trauma with a fracture of the condyle of the occipital bone. Isolated NHN due to mechanical compression by vascular structures may occur due to compression in the carotid space, aneurysms,[
CASE DESCRIPTION
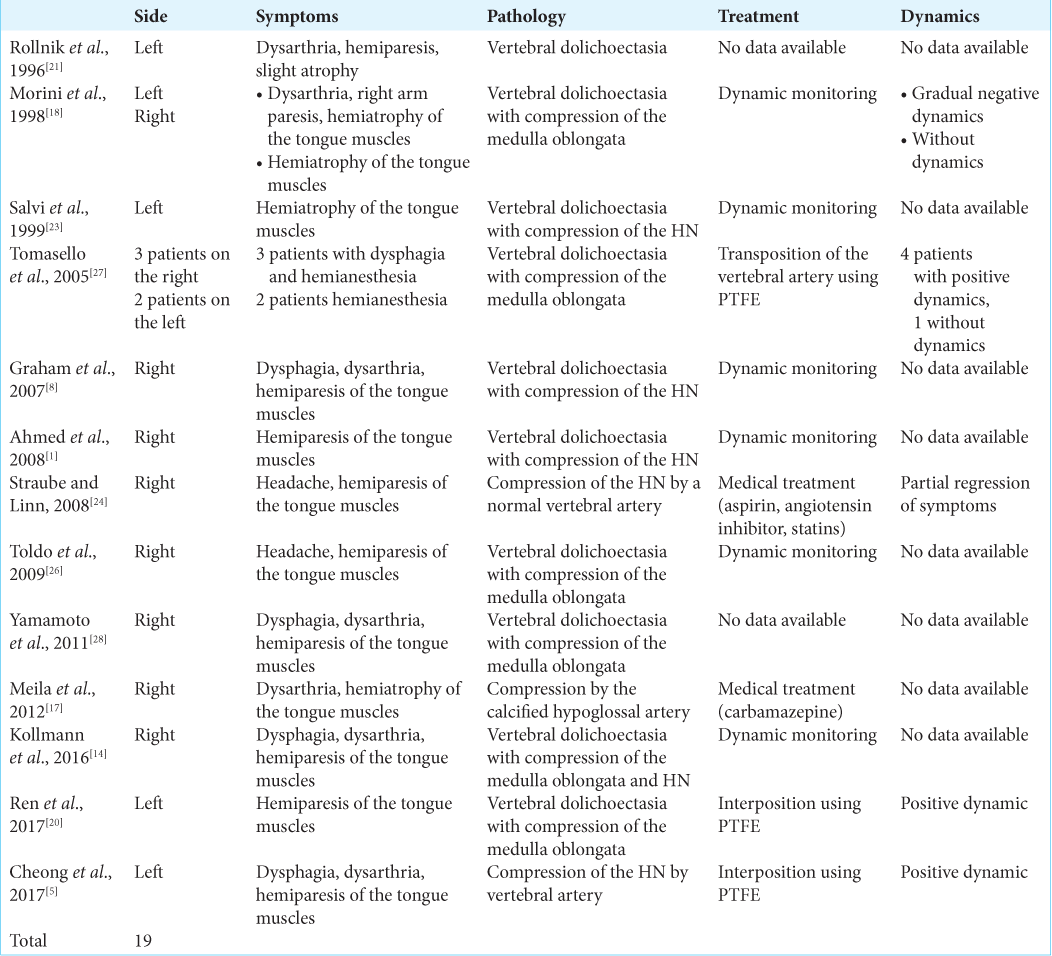
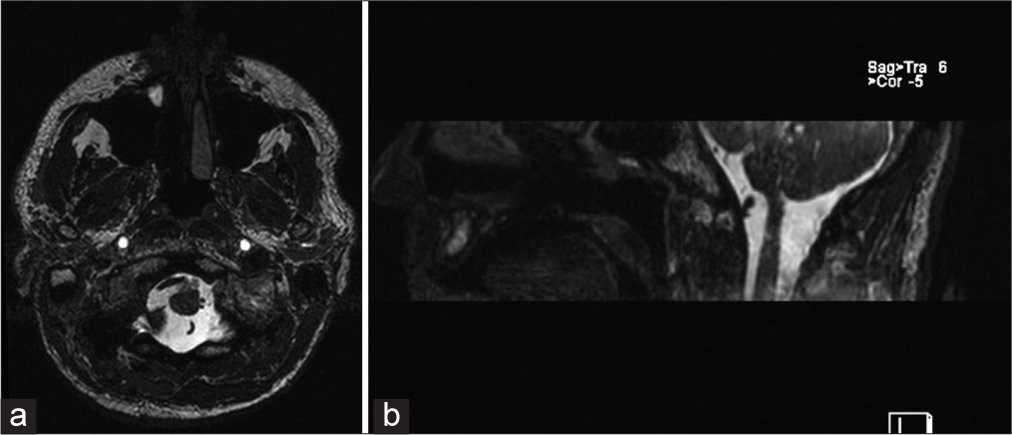
A 28-year-old man in full health condition spontaneously developed weakness of the right side of the tongue with its deviation to the right, 4 years later dysarthria appeared. At the debut of the disease, the patient noted fascial twitching (fasciation?) of half of his tongue. The patient had no history of neck trauma, infectious, and autoimmune diseases. He was treated as an outpatient under the care of a dentist. Results of physical examination of the patient were unremarkable. Neurological examination revealed only peripheral neuropathy of the right side of the tongue with significant atrophy with no signs of deficits on the part of other cranial nerves. A brisk bilateral gag reflex was detected. Cerebellar function was normal, with no symptoms or signs of brainstem compression or increased intracranial pressure. General blood count and C-reactive protein levels excluded an infectious cause. MRI revealed a dolichoectasia of the right VA, which severely compressed the medulla oblongata and roots (XII cranial nerve?) exiting the trunk at the level of the deformity [
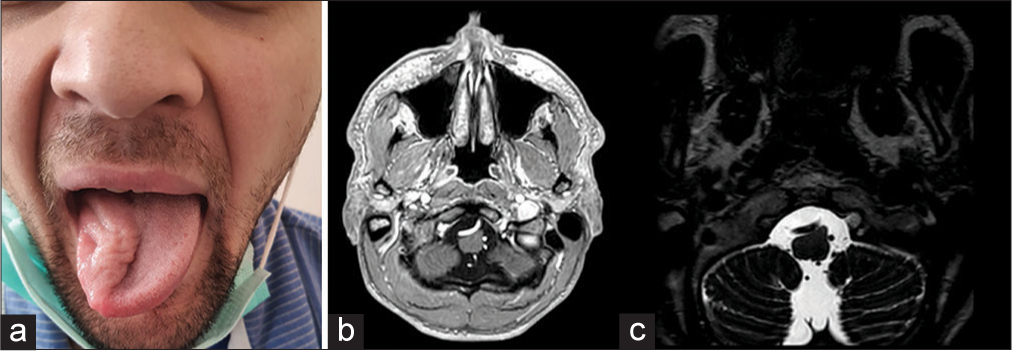
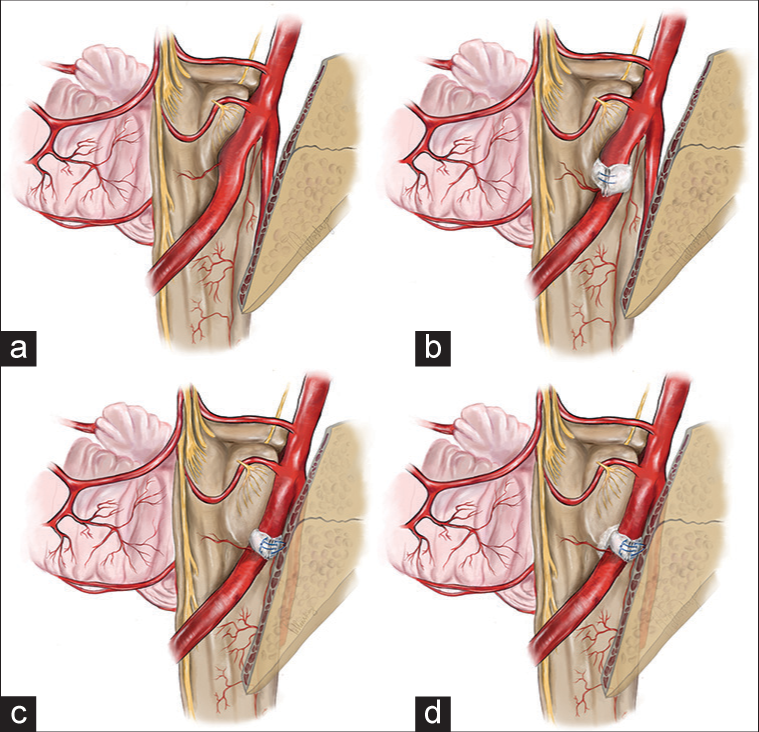
We performed a right-sided “enough” lateral retrosigmoid craniotomy in the position of the patient on the left side [
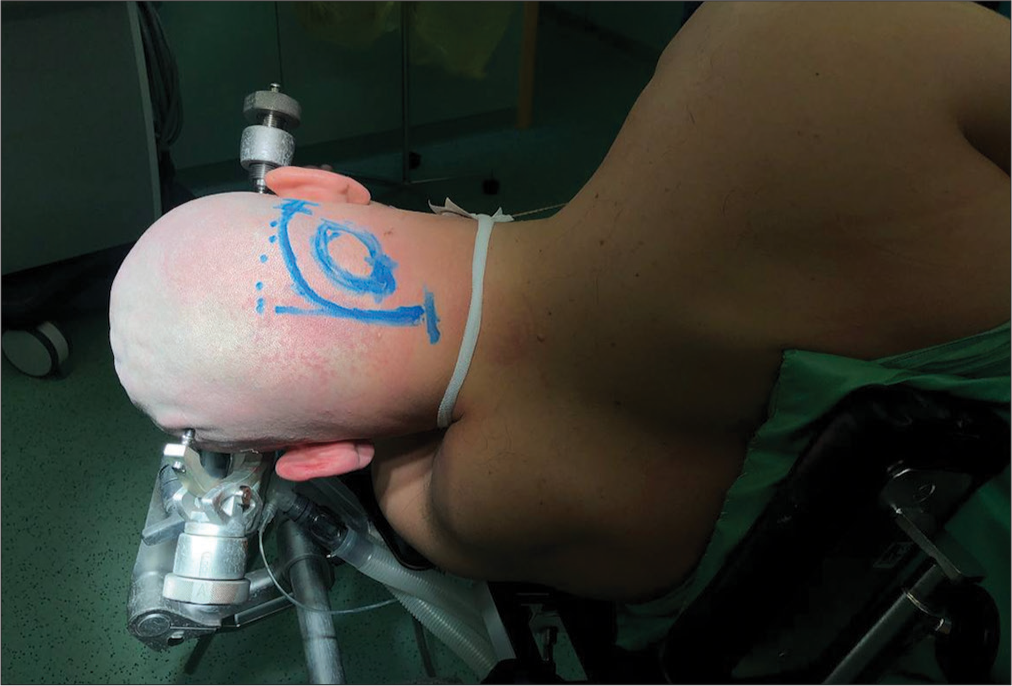
Given the rigidity of the causal vessel in the area of conflict and the predicted low efficiency of interposition, we performed transposition of the vertebral artery by forming a cuff of Teflon material and moving the artery anteriorly by fixing the cuff in the anteromedial direction to the dura mater of the posterior surface of the clivus with single sutures. During transposition, to reduce the stiffness of the main artery, temporary clipping of the vertebral artery was used for 7 min [
Video 1
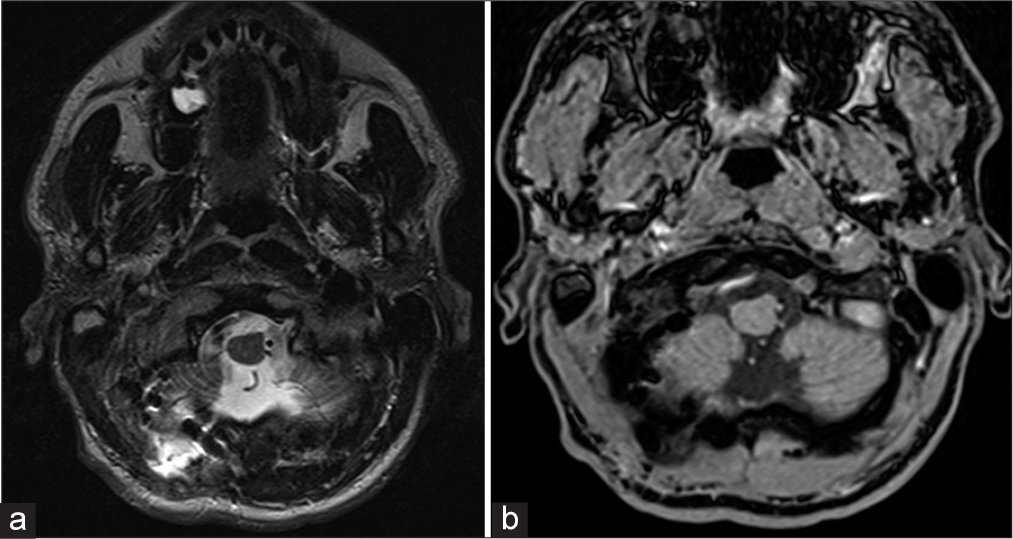
In the 1st day after the surgery, the patient underwent MRI of the brain, visualized the displaced vertebral artery without signs of brainstem compression, the right vertebral artery and other arteries of the posterior cranial fossa contrasted homogeneously, no arterial narrowing or deformities were observed [
At the follow-up examination after 3 months, the atrophy of the tongue muscles had not regressed, but the patient noted an improvement in the mobility of the tongue [
CONCLUSION
Classical methods of interposition using Teflon material in microvascular decompression are mainly used in cases of neurovascular conflicts with small caliber arteries. By definition, this method is of poor efficacy in cases of conflicts caused by large arteries such as the vertebral and basilar arteries, especially in their dolichoectasia and sclerotization. The large diameter of the arteries, thicker, rigid or elastic walls, and high blood pressure in the lumen of these vessels make them less mobile, difficult to shift, and prone to recoiling.
The Teflon gaskets are sometimes not reliable enough to hold and fix such vessels in the optimal position. Therefore, microvascular decompression in cases caused by large caliber arteries is always a difficult technical problem. There are various techniques of using a combination of Teflon (and other synthetic materials) strips and fibrin-thrombin glue,[
The technique of large artery transposition used in this clinical case does not require additional instrumental and material costs and does not disturb the normal anatomy. The advantages of the technique include reliable arterial fixation, and its universality allows its use for neurovascular conflicts on different floors of the posterior cranial fossa.
This clinical case demonstrates the possibility of diagnostic isolated neuropathy of the HN through a detailed clinical and instrumental examination. Compression of the HN at the medullary segment, a rare neurological disease that can be successfully treated by transposition of the vertebral artery using a Teflon cuff.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
Acknowledgment
The authors would like to thank Irina Pollenskaya (drpollenskaya@gmail.com) for providing illustrations for this article.
References
1. Ahmed SN, Aladdin Y, Siddiqi ZA, Khan K. Hypoglossalvertebral entrapment syndrome. Neurology. 2008. 71: 461
2. Bademci G, Batay F, Yaşargil MG. “Triple cross” of the hypoglossal nerve and its microsurgical impact to entrapment disorders. Minim Invasive Neurosurg. 2006. 49: 234-7
3. Bindal S, El Ahmadieh TY, Plitt A, Aoun SG, Neeley OJ, El Tecle NE. Hypoglossal schwannomas: A systematic review of the literature. J Clin Neurosci. 2019. 62: 162-73
4. Cano-Duran AJ, Sanchez Reyes JM, Sevilla MT, Yucumá D. Carotid petrous segment aneurysm presenting as hypoglossal nerve palsy. Neuroradiology. 2021. 63: 447-50
5. Cheong JH, Kim JM, Yang MS, Kim CH. Resolution of isolated unilateral hypoglossal nerve palsy following microvascular decompression of the intracranial vertebral artery. J Korean Neurosurg Soc. 2011. 49: 167-70
6. Choudhri O, Connolly ID, Lawton MT. Macrovascular decompression of the brainstem and cranial nerves: Evolution of an anteromedial vertebrobasilar artery transposition technique. Neurosurgery. 2017. 81: 367-76
7. Gibo H, Marinkovic S, Nikodijevz I, Stimec B, Erden A. The blood supply of the hypoglossal nerve: The microsurgical anatomy of ots cisternal segment. Surg Neurol. 1997. 48: 85-91
8. Graham RM, Thomson EF, Baldwin AJ. Isolated hypoglossal nerve palsy due to a vascular anomaly. Int J Oral Maxillofac Surg. 2007. 36: 759-61
9. Hafkamp HC, Manni JJ, van der Goten A. Unilateral spontaneous dissection of the internal carotid artery presenting as hypoglossal nerve palsy. Eur Arch Otorhinolaryngol. 2004. 261: 405-8
10. He J, Chun J, Lam L, Adlan T. Clival osteomyelitis and hypoglossal nerve palsy-rare complications of Lemierre’s syndrome. BMJ Case Rep. 2015. 2015: bcr2015209777
11. Ichikawa T, Agari T, Kurozumi K, Maruo T, Satoh T, Date I. “Double-stick tape” technique for transposition of an offending vessel in microvascular decompression: Technical case report. Neurosurgery. 2011. 68: 377-82
12. Keane JR. Twelfth-nerve palsy analysis of 100 cases. Arch Neurol. 1996. 53: 561-6
13. Kesserwani H. Isolated palsy of the cisternal segment of the hypoglossal nerve due to arterial dissection of the V4 segment of the vertebral artery: A case report with a side note on nerve trunk ischemia. Cureus. 2020. 12: e9930
14. Kollmann P, Raucq E, Fransen P. Isolated hypoglossal nerve paralysis and hypoglossal vertebral entrapment syndrome. Acta Neurol Belg. 2017. 117: 377-80
15. Mahadevappa K, Chacko T, Nair AK. Isolated unilateral hypoglossal nerve palsy due to vertebral artery dissection. Clin Med Res. 2012. 10: 127-30
16. McKeon A, Murphy S, McNamara B, Ryder DO, Galvin RJ. Isolated hypoglossal nerve palsy due to compression by a dissecting vertebral artery. Eur Neurol. 2005. 53: 162-4
17. Meila D, Wetter A, Brassel F, Nacimiento W. Intermittent hypoglossal nerve palsy caused by a calcified persistent hypoglossal artery: An uncommon neurovascular compression syndrome. J Neurol Sci. 2012. 323: 248-9
18. Morini A, Rozza L, Manera V, Buganza M, Tranquillini E, Orrico D. Isolated hypoglossal nerve palsy due to an anomalous vertebral artery course: Report of two cases. 1998. 19: 379-82
19. Nonaka Y, Hayashi N, Matsumae M, Fukushima T. Wedge-technique for transposition of the vertebral artery in microvascular decompression for hemifacial spasm: Technical nuances and surgical outcomes. Acta Neurochirurg. 2019. 161: 1435-42
20. Ren J, Sun H, Diao Y, Niu X, Wang H, Wei Z, Yuan F. Successful treatment with microvascular decompression surgery of a patient with hemiparesis caused by vertebral artery compression of the medulla oblongata: Case report and review of the literature. World Neurosurg. 2017. 108: 994.e11-9
21. Rollnik JD, Sindern E, Mosler F, Im Spring B, Malin JP. Isolated peripheral hypoglossal palsy caused by a kinking of the left vertebral artery (hypoglossal vertebral entrapment syndrome). Eur Neurol. 1996. 36: 324-5
22. Sai Kiran NA, Sivaraju L, Furtado SV, Vidyasagar K, Raj V, Aryan S. Far lateral approach without occipital condylar resection for intradural ventral/ventrolateral foramen magnum tumors and aneurysms of V4 segment of vertebral artery: Review of surgical results. Clin Neurol Neurosurg. 2020. 197: 106163
23. Salvi F, Mascalchi M, Plasmati R, Tugnoli V, De Grandis D. Hypoglossal vertebral entrapment syndrome. Muscle Nerve. 1999. 22: 288-9
24. Straube A, Linn J. Unilateral headache attacks and ipsilateral atrophy of the tongue due to neurovascular compression of the hypoglossal nerve. Cephalalgia. 2008. 28: 996-8
25. Thompson EO, Smoker WR. Hypoglossal nerve palsy: A segmental approach. Radiographics. 1994. 14: 939-58
26. Toldo I, Manara R, Sartori S, Suppiej A, Drigo P. Unilateral hypoglossal nerve palsy due to neurovascular conflict in a child. Brain Dev. 2009. 31: 461-4
27. Tomasello F, Alafaci C, Salpietro FM, Longo M. Bulbar compression by an ectatic vertebral artery: A novel neurovascular construct relieved by microsurgical decompression. Neurosurgery. 2005. 56: 117-24
28. Yamamoto M, Suzuki K, Takekawa H, Hirata K. Isolated hypoglossal nerve palsy caused by neurovascular compression. Intern Med. 2011. 50: 2701-2