- Department of Surgery, Santa Casa de Sao Paulo School of Medical Sciences, Rua Dr. Cesário Motta Júnior, São Paulo, Brazil.
DOI:10.25259/SNI_224_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ricardo Salemi Riechelmann, Leonardo Henrique Rodrigues, Tiago Marques Avelar, Paulo Adolfo Xander, Guilherme Henrique da Costa, Luiz Fernando Cannoni, Guilherme Brasileiro de Aguiar, Jose Carlos Veiga. Isolated neuroparacoccidioidomycosis as a pseudotumoral lesion in the absence of systemic disease. 13-Jun-2020;11:151
How to cite this URL: Ricardo Salemi Riechelmann, Leonardo Henrique Rodrigues, Tiago Marques Avelar, Paulo Adolfo Xander, Guilherme Henrique da Costa, Luiz Fernando Cannoni, Guilherme Brasileiro de Aguiar, Jose Carlos Veiga. Isolated neuroparacoccidioidomycosis as a pseudotumoral lesion in the absence of systemic disease. 13-Jun-2020;11:151. Available from: https://surgicalneurologyint.com/surgicalint-articles/10080/
Abstract
Background: Paracoccidioidomycosis (PCM) is a systemic, progressive, noncontagious, and often chronic disease caused by the fungus Paracoccidioides brasiliensis that rarely affects the central nervous system (CNS). The condition is usually treated using antifungal drugs, and some cases may require surgery.
Case Description: A 55-year-old man, a smoker, without known comorbidities, was referred to the neurosurgery team with a history of a single epileptic seizure a week before hospital admission followed by progressive right- sided hemiparesis. Head computed tomography and brain magnetic resonance imaging showed an intra-axial expansive lesion affecting the left parietal lobe, associated with extensive edema and a regional compressive effect producing slight subfalcine herniation that was initially managed as an abscess. After the failure of antibiotic treatment, the patient underwent a neurosurgical procedure for excision of the lesion. Histopathological analysis revealed that it was PCM and there was no evidence of impairment of other systems due to the disease.
Conclusion: PCM can be a serious, debilitating disease and is potentially fatal. Although isolated CNS involvement is rare, it must be considered, especially in endemic areas, as late diagnosis and treatment severely decreases good outcome rates.
Keywords: Central nervous system infections, Neurosurgical procedures, Paracoccidioidomycosis
INTRODUCTION
Paracoccidioidomycosis (PCM) is a systemic, progressive, noncontagious, and often chronic disease caused by the fungus Paracoccidioides brasiliensis that affects mainly Latin American men engaged in agriculture and in contact with soil.[
CASE REPORT
A 55-year-old man, a recycler, smoker, and alcoholic, without known comorbidities, was referred to the neurosurgery division with a history of a single epileptic seizure 1 week before hospital admission followed by progressive right-sided hemiparesis. On initial evaluation, muscle strength was graded as III and IV in the proximal and distal right upper limb, respectively, and IV in the right lower limb. There was also tactile hypoesthesia in the right hemibody. On visual examination, the patient’s remaining teeth were in a very bad hygienic condition, and most of them were missing. Head computed tomography (CT) and brain magnetic resonance imaging (MRI) showed an intra-axial expansive lesion affecting the left parietal lobe, associated with extensive edema and a regional compressive effect producing slight subfalcine herniation. A pyogenic abscess was the main diagnostic hypothesis and given the poor oral condition and lack of other findings, the primary infection site was presumed to be odontogenic.
Antibiotic therapy (ceftazidime + metronidazole + vancomycin) and administration of dexamethasone were then initiated. Approximately 10 days later, the patient developed a high fever followed by one generalized tonic-clonic seizure despite the use of phenytoin. Within a few hours, he suffered a cardiopulmonary arrest and only returned to spontaneous circulation after 38 min of cardiopulmonary resuscitation and was admitted to the ICU, where he managed regain consciousness overtime, while maintaining previous deficits and radiologic findings.
After a new brain MRI showed lesion growth despite the antibiotic therapy for 40 days, stereotactic surgical treatment was indicated and successfully performed, but the histopathological analysis was inconclusive. A control CT scan showed a small reduction in perilesional edema and signs of a remnant lesion.
The patient was discharged without antibiotic therapy after 3 months of hospitalization and maintained clinical stability in a follow-up evaluation 20 days later with mild improvement in the right hemibody strength and a single focal seizure episode.
Three months later, the patient returned with an increase in the frequency of focal seizures and an increase in the remnant lesion, observed by a CT and MRI performed on readmission [
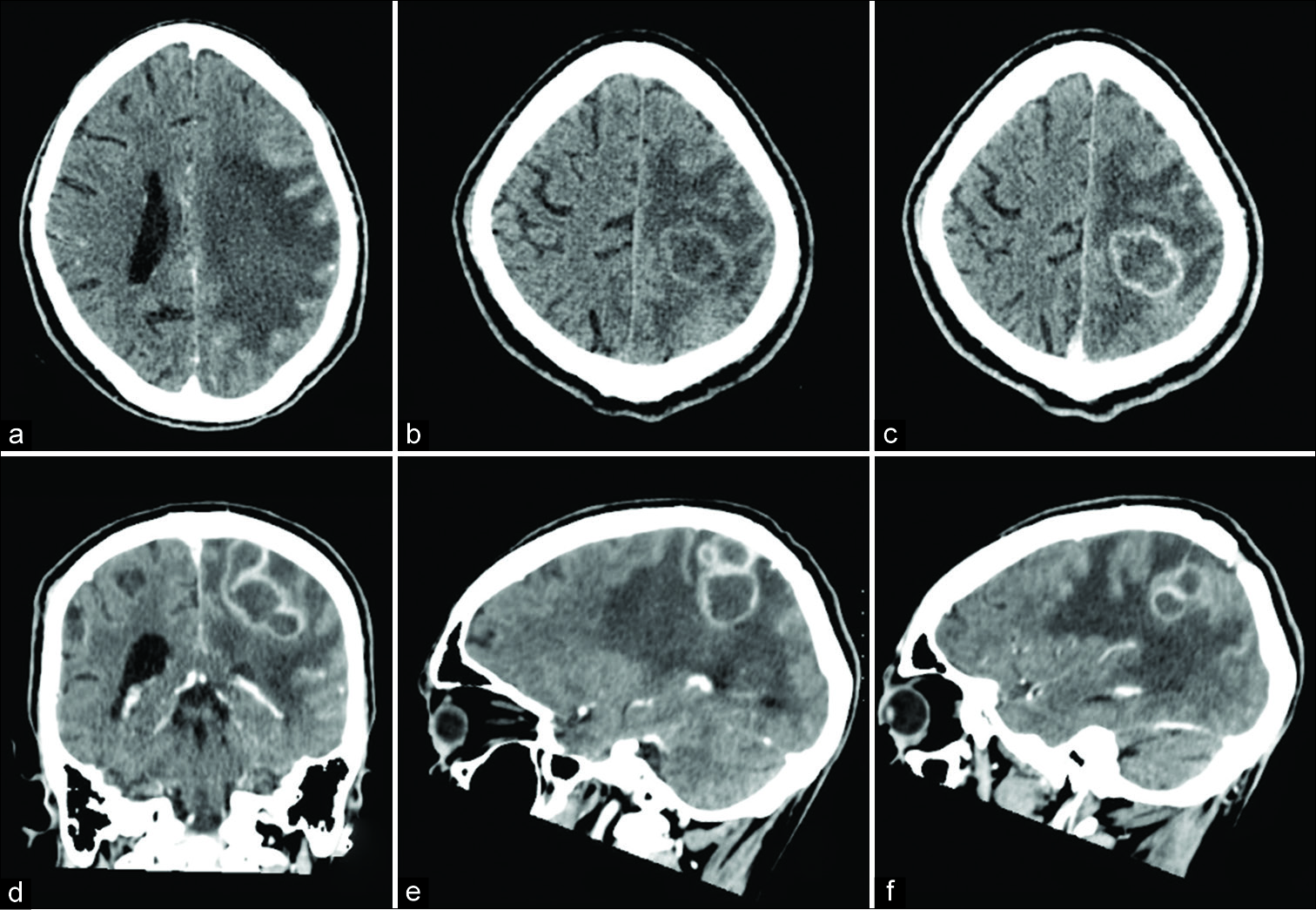
Figure 1:
Computed tomography scan before (a and b) and after (c-f) contrast injection. Significant perilesional edema and isodense peripheral aspect of multinodular subcortical left parietal lesion with hypodense content to normal parenchyma with moderate mass effect to the left lateral ventricle (a and b). Ring peripheral postcontrast enhancement in axial, coronal, and sagittal views (d-f).
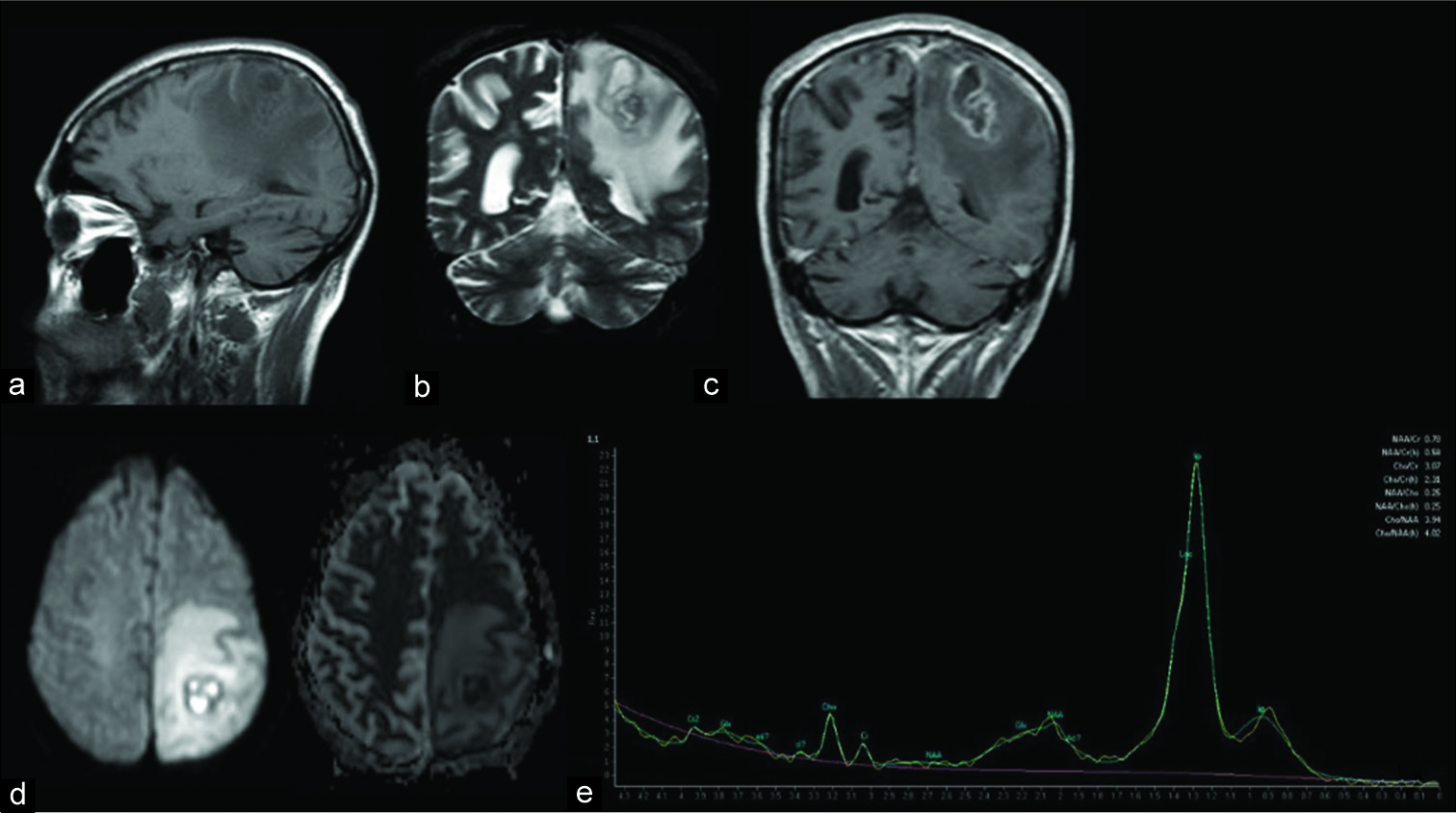
Figure 2:
Magnetic resonance imaging findings: perilesional edema, iso/hyperintense periphery with hypointense content on T1WI (a), hypointense periphery with hyperintense content on T2WI (b), postcontrast periphery enhancement (c), restricted diffusion (d), and lipid peak at 1.3 ppm in spectroscopy (e).
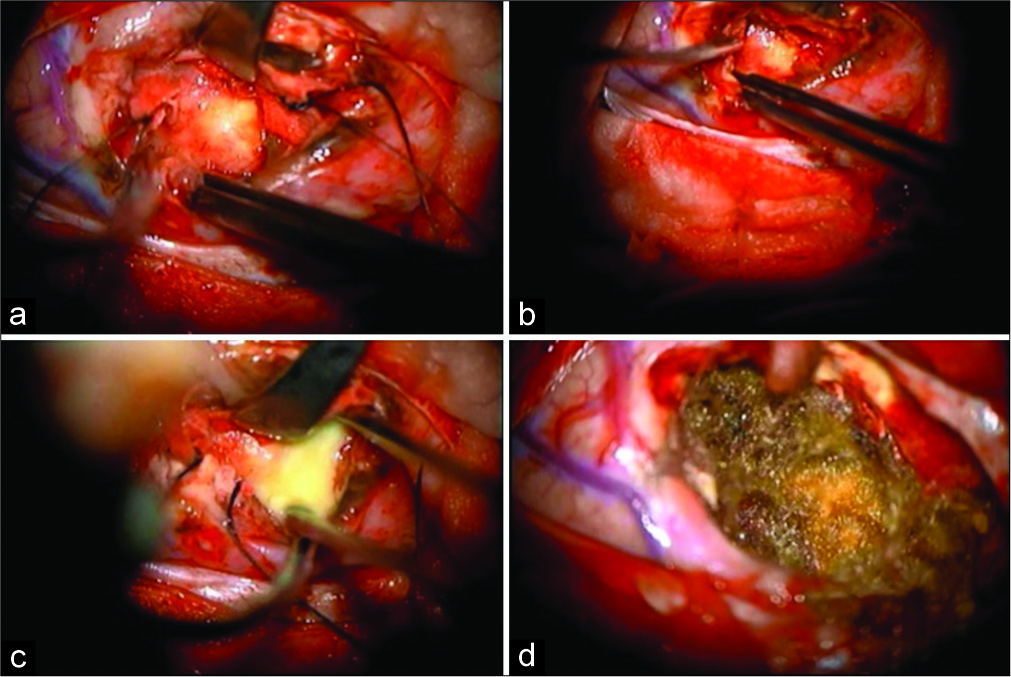
Figure 3:
Intraoperative findings of the lesion. (a) Firm capsulated lesion. (b) Microdissection in the cleavage plane between the capsule and with matter tissue. (c) Break of the capsule with liquefactive necrotic material leakage. (d) Final aspect with resection cavity lined by fibrillar hemostatic.
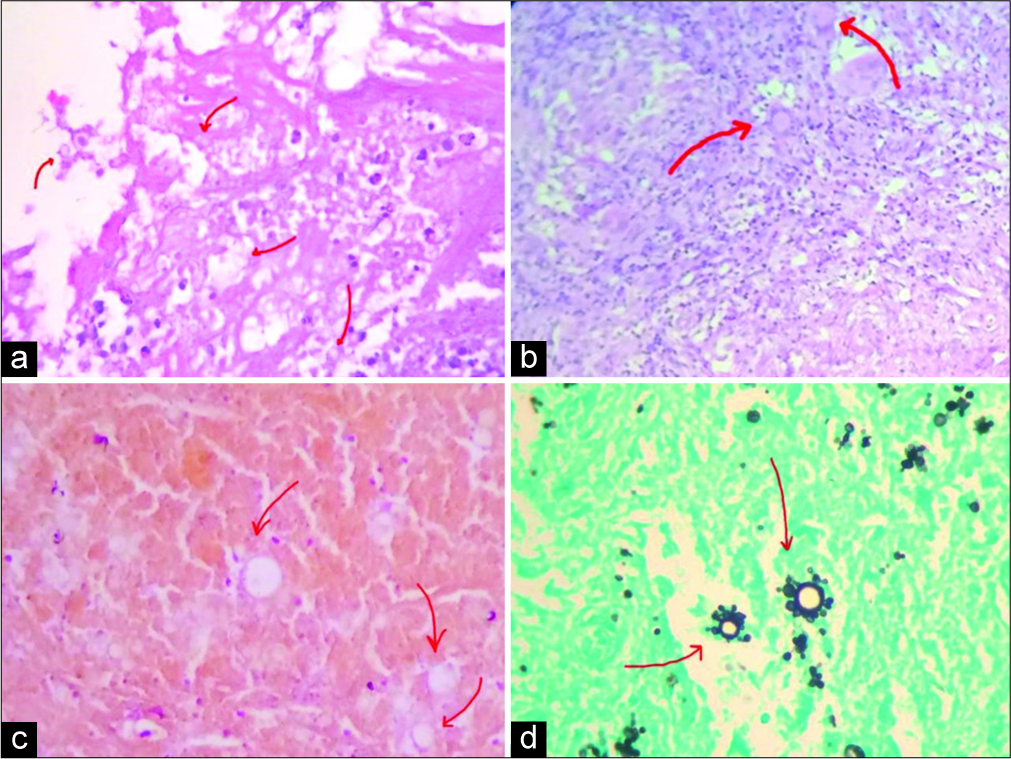
The anatomopathological study showed brain tissue with an inflammatory process characterized by granulomas with multinucleated giant cells next to extensive areas of necrosis. In the middle of the process and, sometimes, in the giant cells, histological examination using the Grocott-Gomori’s methenamine silver nitrate stain method showed highly specific signs of cerebral PCM similar to a ship’s wheel or Mickey Mouse ears [
Figure 4:
Histological study: (a-c) brain parenchyma with reactive infiltrate of lymphocytes and giant cells with central necrosis gliosis (red arrows) (Hematoxylin and Eosin); (d) presence of characteristic helm-shaped yeasts (multiple budding) compatible with paracoccidioidomycosis (Gomori’s methenamine silver stain).
Itraconazole was then prescribed after the suspension of the antibiotic therapy. The patient was discharged from hospital and after responding well to treatment and making a partial recovery of his neurological deficits.
DISCUSSION
Brazil is the country with the most cases of PCM in Latin America with a prevalence estimated at between 5.6 and 17.5%, with an age peak of 30–50 years of age.[
NPCM can be manifested as meningitis, meningoencephalitis, meningoradiculitis, or pseudotumoral forms,[
The most important differential diagnosis is tuberculosis, a disease that may coexist with the mycosis in 8% of patients. Other diseases that should be considered are cancer and neoplastic disorders (including lymphoma), histoplasmosis, leishmaniasis, leprosy, and syphilis. Since symptoms and radiological findings are nonspecific,[
Findings in MRI studies reveal an iso- to hyperintense signal on T1WI sequences and a hypointense signal on T2WI sequences at the periphery of the lesions, frequently associated with significant perilesional edema (hypointense on T1WI and hyperintense on T2WI), as demonstrated in the present case. Contrast (gadolinium) enhancement is also present, mainly as a peripheral ring enhancement but also a heterogeneous enhancement. The interior of the lesions is hypointense on T1WI and hyperintense on T2WI. As in granulomatous diseases such as neurotuberculosis, restricted diffusion is usually absent in NPCM due to solid caseation and associated fibrosis, but can be present, as happened in this case. MRI spectroscopy showed lipid peaks (1.3 ppm) that could be useful to differentiate NPCM from other lesions such as pyogenic abscesses (where peaks of succinate at 2.4 ppm, acetoacetate at 1.9 ppm, and alanine at 1.4 ppm may be seen).[
Treatment for PCM is mainly clinical with antifungal drugs such as trimethoprim/sulfamethoxazole (with low toxicity and easily availability)[
The prognosis is not very good, especially in disseminated forms and cases of late diagnosis, in which mortality can reach up to 20%.[
CONCLUSION
Paracoccidioidomycosis can be a serious debilitating disease and potentially fatal if untreated, especially because patients are often immunodepressed. Early diagnosis is, therefore, necessary for a good response to treatment and better prognosis. PCM in the CNS is not a common condition but the possibility of it occurring in the CNS should not be overlooked by clinicians, neurologists, and neurosurgeons, especially those who work in endemic areas as there is a specific treatment and real potential to cure the condition. Late diagnosis and treatment severely decrease good outcome rates. Our case progressed well, mainly because of rapid hospital admission and surgical resection of the lesion. Drug therapy was promptly initiated and adjusted after the biopsy results for the lesion were available.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Benard G, Campos AF, Netto LC, Goncalves LG, Machado LR, Mimicos EV. Treatment of severe forms of paracoccidioidomycosis: Is there a role for corticosteroids?. Med Mycol. 2012. 50: 641-8
2. da Silva CE, Cordeiro AF, Gollner AM, Cupolilo SM, Quesado-Filgueiras M, Curzio MF. Paracoccidioidomycosis of the central nervous system: Case report. Arq Neuropsiquiatr. 2000. 58: 741-7
3. da Silva SH, Colombo AL, Blotta MH, Queiroz-Telles F, Lopes JD, de Camargo ZP. Diagnosis of neuroparacoccidioidomycosis by detection of circulating antigen and antibody in cerebrospinal fluid. J Clin Microbiol. 2005. 43: 4680-3
4. de Almeida SM, Salvador GL, Roza TH, Izycki LF, Dos Santos I, Aragao A. Geographical evaluation of neuroparacoccidioidomycosis and paracoccidioidomycosis in Southern Brazil. Mycoses. 2018. 61: 587-93
5. de Medeiros FC, de Moraes AC, Nunes MB, Dellaretti M. Neuroparacoccidioidomycosis mimicking brain tumor in an immunocompetent patient. Rev Neurol (Paris). 2018. 174: 268-71
6. Elias J, dos Santos AC, Carlotti CG, Colli BO, Canheu A, Matias C. Central nervous system paracoccidioidomycosis: Diagnosis and treatment. Surg Neurol. 2005. 63: S13-21
7. Isolan G, Vieira D, Hehn F, Antunes AC. Paracoccidioidomycosis simulating brain tumor. Surg Neurol Int. 2014. 5: 134-
8. Jorge LA, Yamashita S, Trindade AP, Resende LA, Zanini MA, Xavier JC. Pseudotumoral neuroparacoccidioidomycosis of the posterior fossa: A case report and review of the literature. Surg Neurol Int. 2017. 8: 76-
9. Morais MV, Georgeto SM, Haddad ML, Amorim JG, Scaliante LG, Paula AL. Neuroparacoccidioidomycosis: Case report and literature review. Arq Bras Neurocir Braz Neurosurg. 2018. 37: 134-9
10. Naik V, Ahmed FU, Gupta A, Garg A, Sarkar C, Sharma B. Intracranial fungal granulomas: A single institutional clinicopathologic study of 66 patients and review of the literature. World Neurosurg. 2015. 83: 1166-72
11. Ortiz S, Wiesner S, Cataño JC. An immunocompetent 49-year-old man with a disseminated infection. Eur J Intern Med. 2016. 34: e5-6
12. Paniago AM, de Oliveira PA, Aguiar ES, Aguiar JI, da Cunha RV, Leme LM. Neuroparacoccidioidomycosis: Analysis of 13 cases observed in an endemic area in Brazil. Trans R Soc Trop Med Hyg. 2007. 101: 414-20
13. Reis F, Collier PP, Souza TF, Lopes GP, Bronzatto E, Junior NA. Neuroparacoccidioidomycosis (NPCM): Magnetic resonance imaging (MRI) findings. Mycopathologia. 2013. 175: 181-6
14. Tristano AG, Chollet ME, Willson M, Perez J, Troccoli M. Central nervous system paracoccidioidomycosis: Case report and review. Invest Clin. 2004. 45: 277-88