- Wolfson School of Medicine, University of Glasgow, Scotland, United Kingdom,
- Department of Neurosurgery, National Hospital and Medical Centre, Lahore, Punjab, Pakistan,
- School of Medicine, King Edward Medical University, Lahore, Punjab, Pakistan,
- School of Medicine, Allama Iqbal Medical College, Lahore, Punjab, Pakistan,
- Department of Infectious Diseases, National Hospital and Medical Centre, Lahore, Punjab, Pakistan.
Correspondence Address:
Mohammad Ashraf, Wolfson School of Medicine, University of Glasgow, Scotland, United Kingdom.
DOI:10.25259/SNI_334_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohammad Ashraf1, Syed Shahzad Hussain2, Minaam Farooq3, Laveeza Fatima4, Nadia Majeed5, Naveed Ashraf2. Isolated subdural hematoma due to dengue hemorrhagic fever: Surgical intervention and review of the literature. 10-Jun-2022;13:244
How to cite this URL: Mohammad Ashraf1, Syed Shahzad Hussain2, Minaam Farooq3, Laveeza Fatima4, Nadia Majeed5, Naveed Ashraf2. Isolated subdural hematoma due to dengue hemorrhagic fever: Surgical intervention and review of the literature. 10-Jun-2022;13:244. Available from: https://surgicalneurologyint.com/surgicalint-articles/11647/
Abstract
Background: Central nervous system (CNS) complications of dengue fever, a mosquito-borne single standard RNA virus illness, are reported in
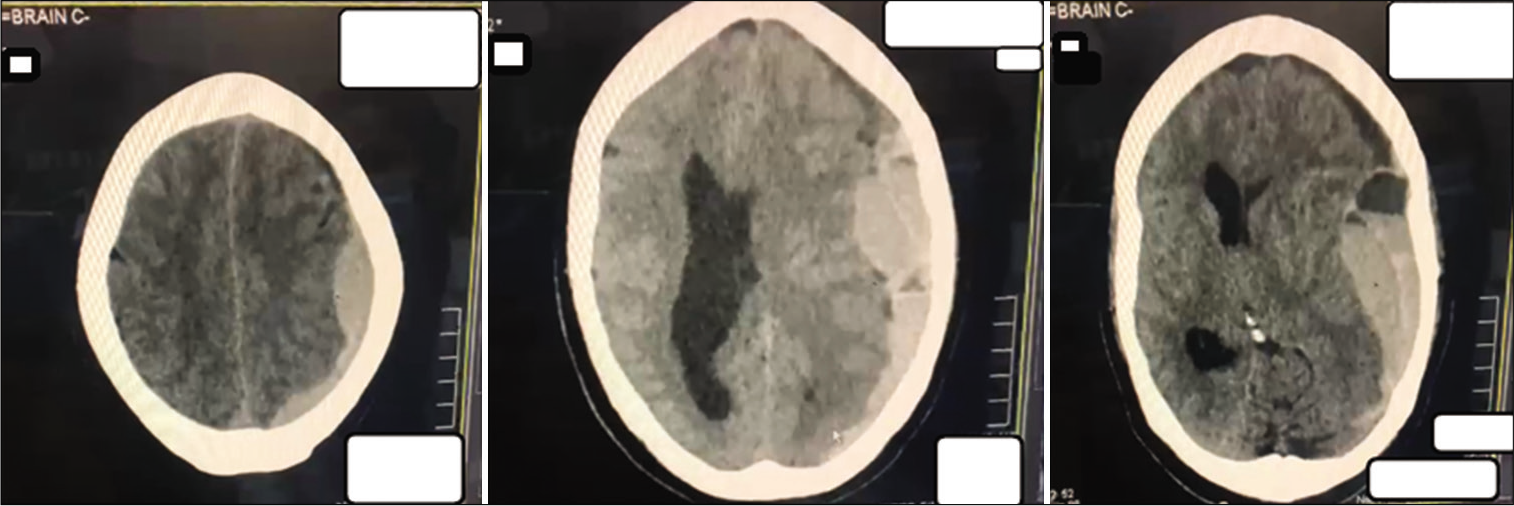
Case Description: A 65-year-old male patient was admitted with high-grade febrile illness and diagnosed with dengue. The patient had no focal neurology and was managed adequately following the primary survey on admission but, then, developed severe thrombocytopenia and eventually the critical phase of dengue illness. On the 5th admission day, the patient collapsed. Glasgow Coma Score was 3/15 with bilaterally dilated, fixed pupils. Immediate computed tomography head revealed a large left SDH with a significant midline shift. SDH was emergently evacuated with two units of platelets transfused peroperatively and two additional units postoperatively. Thrombocytopenia resolved within 48 h, and interval scanning showed gradual resolution of SDH. The patient was discharged 18 days later. Five months later, on follow-up, the patient is well with mild left-sided weakness and an Extended Glasgow Outcome Score of 7.
Conclusion: Isolated SDH is a rare but life-threatening hemorrhagic complication of DHF. Even in the critical phase of illness, with severe thrombocytopenia, surgical evacuation should be considered if the SDH is present in isolation, within an accessible area, and can be operated on immediately.
Keywords: Dengue, Dengue hemorrhagic fever, Dengue shock syndrome, Neurological complication, Subdural hematoma
INTRODUCTION
Dengue is a single-stranded RNA viral infection transmitted by the bite of Aedes aegypti or albopictus mosquitoes.[
Neurological or central nervous system (CNS) involvement/ complications of dengue are exceedingly rare, with a reported incidence of <1%.[
CASE PRESENTATION
A 65-year-old male presented to the emergency department on the night of October 19 reporting high-grade fever and body aches for 3 days. The patient acutely developed dizziness and abdominal pain and complained of a new-onset headache. On arrival, he was hypotensive, tachycardic, with a temperature of 39.8°C, and oliguric. His Glasgow Coma Score (GCS) was 15/15, and examination revealed no focal neurology. Dengue fever was suspected, and an ultrasound revealed pericholecystic edema and free fluid in the patient’s abdomen. The patient was managed adequately with fluid resuscitation, admitted for observation, and managed according to our national guidelines by the Punjab Dengue Expert Advisory Group.[
Dengue test revealed
Soluble nonstructural protein 1 (test method – dengue NS 1 Antigen, detection window day 1–5) Dengue viral RNA (test method – dengue real-time polymerase chain reaction, detection window day 1–5) Dengue IgM antibodies indicating an acute or recent infection Dengue IgG in high levels indicating a secondary infection.
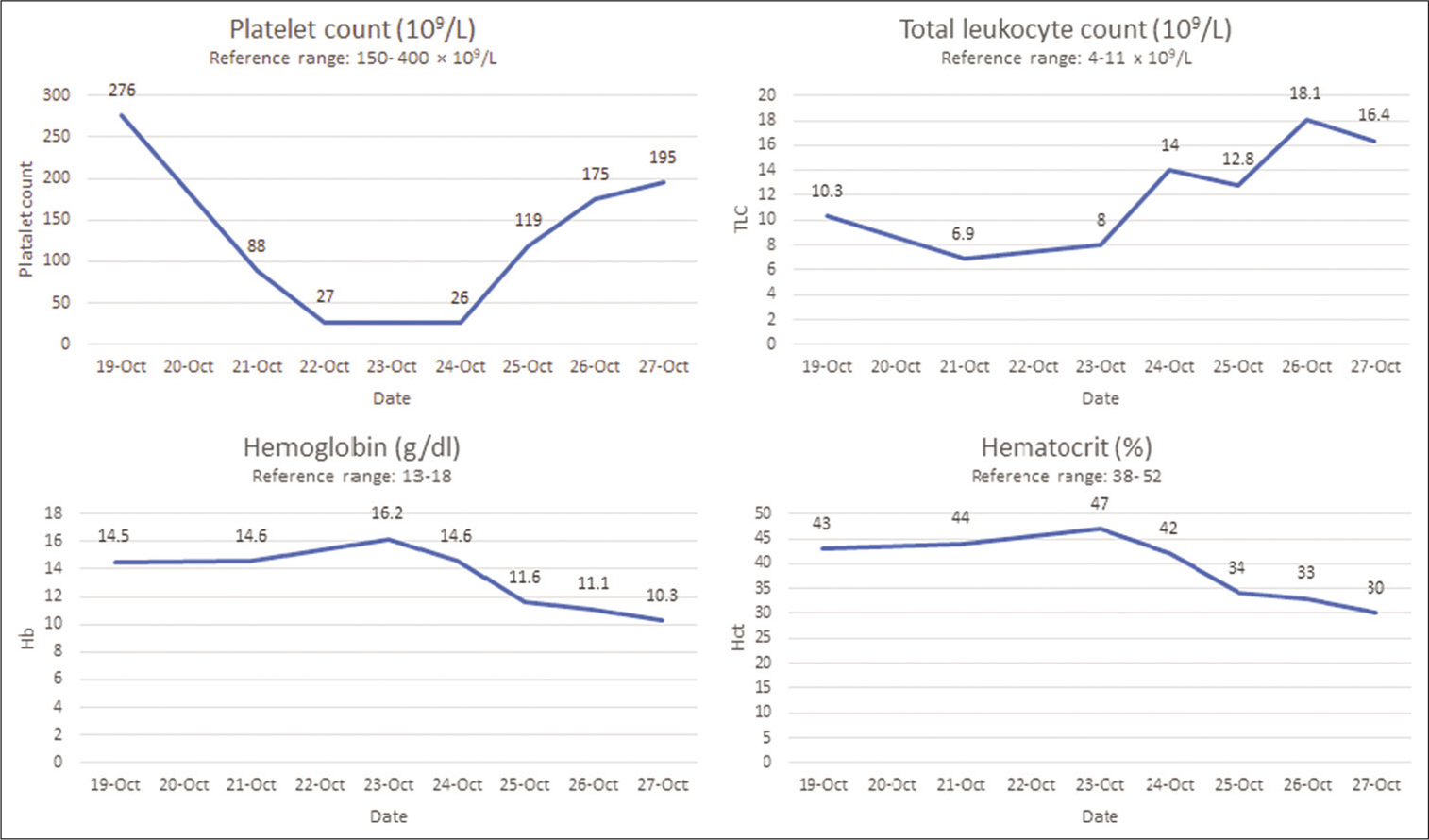
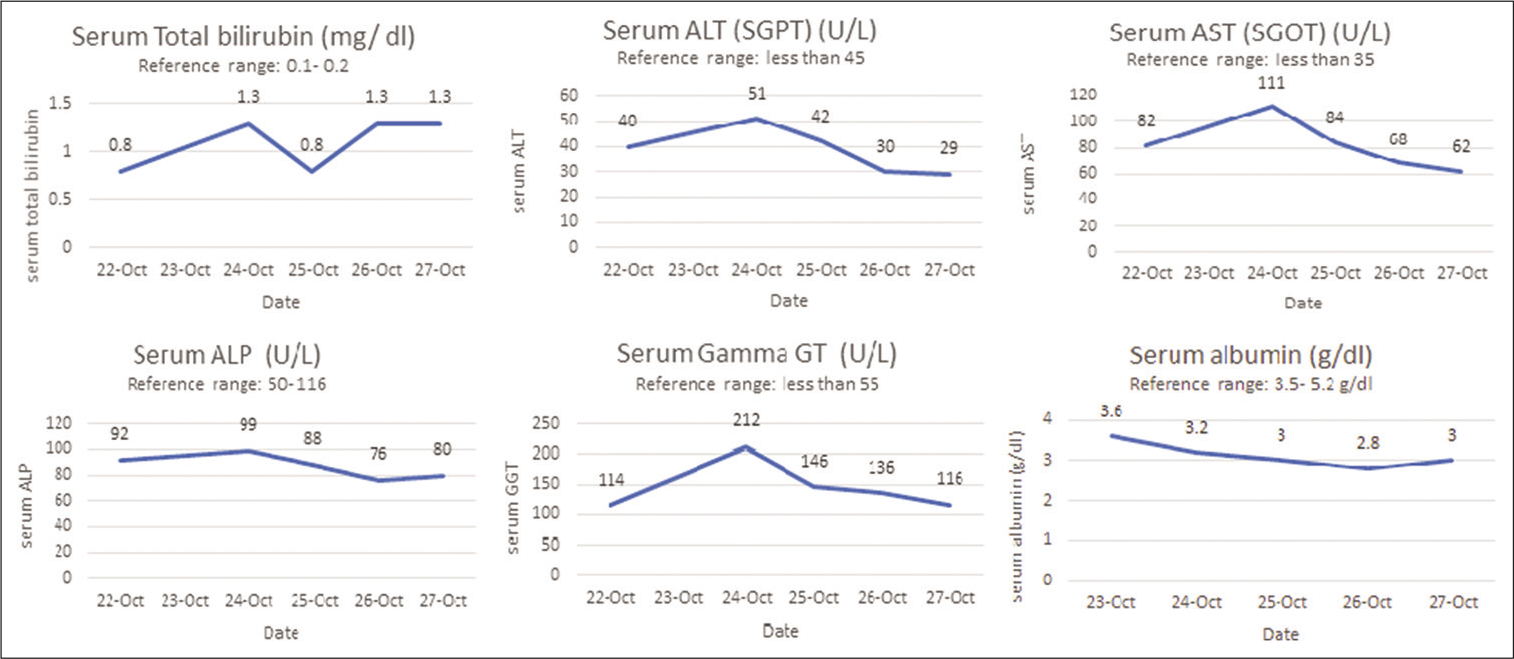
The patient was clinically stable over the next 3 days but developed a sharp progressive decline in his platelet count and eventually severe thrombocytopenia. Changes in platelet counts and deterioration of important biochemical parameters are summarized in
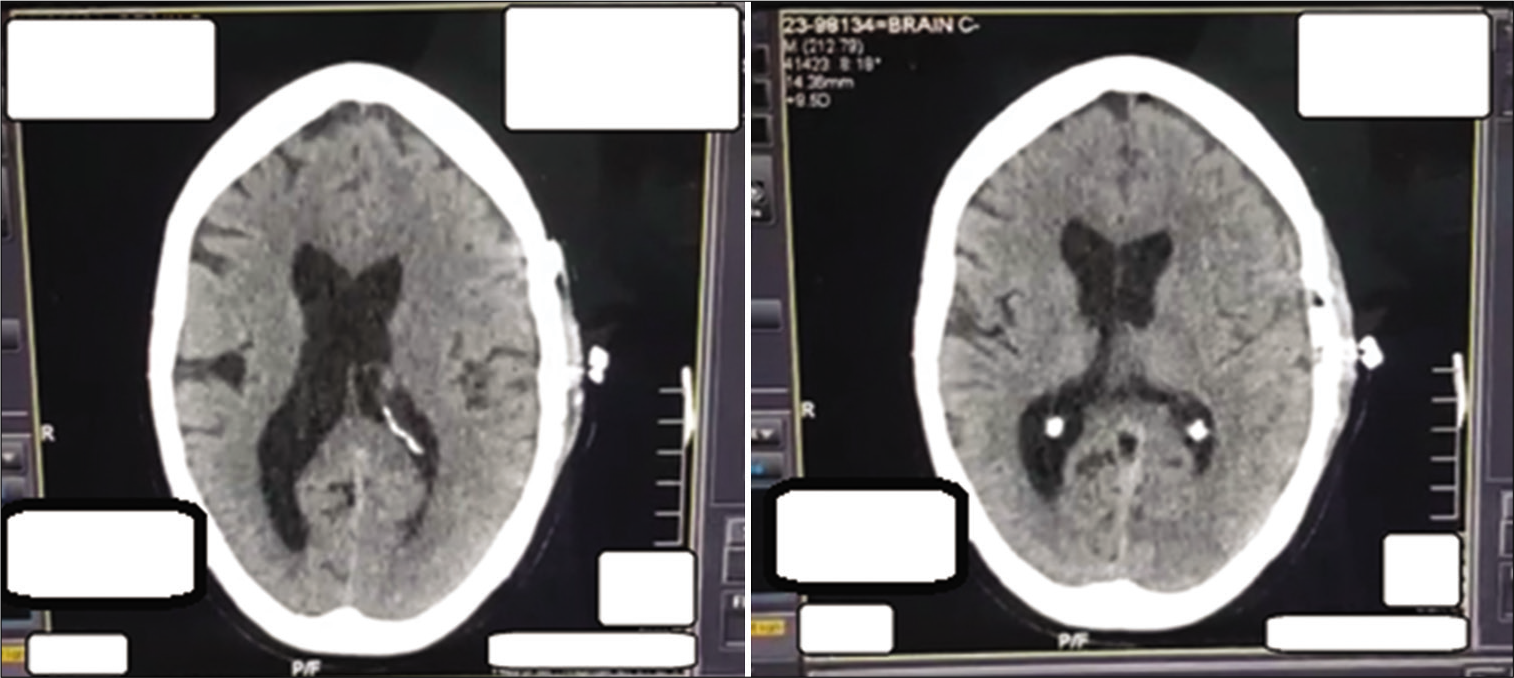
The patient developed signs of a chest infection on October 28 and was diagnosed with ventilator-associated pneumonia, for which he was treated. Compared to the previous interval scans, his final CT scan performed on November 3 showed complete resolution of the subdural hemorrhage and midline shift and resorption of residual air [
DISCUSSION
Whether dengue fever will be asymptomatic or manifest as a mild infection or severe disease is determined by a complex interaction of the hosts’ immune system to the virus. The immunological basis of DHF and DSS is secondary infection with a different serotype with an antibody-dependent enhancement of the dengue virus infection, whereby nonprotective antibodies against the virus bind to the Fc receptor of immune cells.[
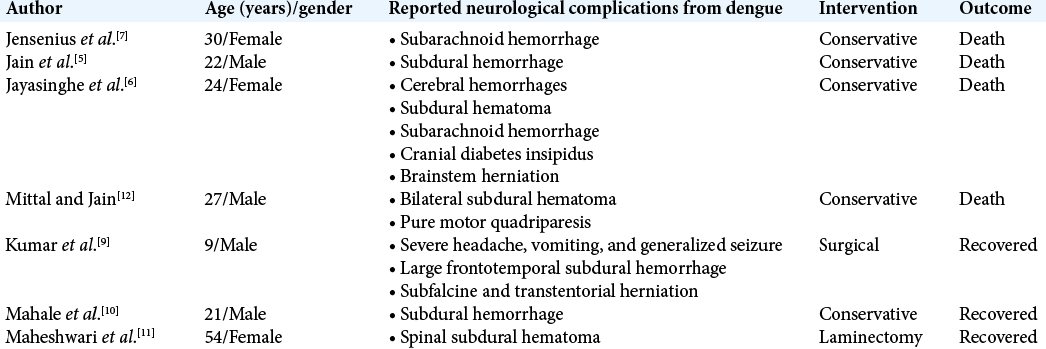
Cases reporting neurological complications from dengue are sparse. The previous case reports include an isolated fatal subarachnoid hemorrhage (postmortem confirming no previous intracranial arterial aneurysms) or isolated SDHs.[
According to the literature, the critical phase of dengue is characterized by the abatement of temperature, which reduces from a high-grade fever to approximately 37.5–38°C and is seen on days 3 to 7 of the illness.[
CONCLUSION
Severe forms of dengue illness are common in areas, where the virus is an endemic or epidemic. Hemorrhagic complications greatly increase mortality, and while neurological complications, including CNS hemorrhage, are very rare, they can be fatal. There should be a high clinical suspicion of intracranial hemorrhage when neurological complications manifest, and urgent neurosurgical review should be sought. Timely, surgical intervention in select cases such as ours may change the outcome to a favorable one in this rare but severe complication of dengue.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alejandria MM. Dengue haemorrhagic fever or dengue shock syndrome in children. BMJ Clin Evid. 2015. 2015: 0917
2. Dengue Expert Advisory Group Guidlines. Available from: https://deag.punjab.gov.pk [Last accessed on 2022 Apr 06].
3. Haider Z, Ahmad FZ, Mahmood A, Waseem T, Shafiq I, Raza T. Dengue fever in Pakistan: a paradigm shift; changing epidemiology and clinical patterns. Perspect Public Health. 2015. 135: 294-8
4. Hasan S, Jamdar SF, Alalowi M, Al Ageel Al Beaiji SM. Dengue virus: A global human threat: Review of literature. J Int Soc Prev Community Dent. 2016. 6: 1-6
5. Jain N, Gutch M, Kumar V, Naik AK. A fatal combo of dengue shock syndrome with acute subdural hematoma. Neurol India. 2012. 60: 105-6
6. Jayasinghe NS, Thalagala E, Wattegama M, Thirumavalavan K. Dengue fever with diffuse cerebral hemorrhages, subdural hematoma and cranial diabetes insipidus. BMC Res Notes. 2016. 9: 265
7. Jensenius M, Berild D, Ormaasen V, Maehlen J, Lindegren G, Falk KI. Fatal subarachnoidal haemorrhage in a Norwegian traveller with dengue virus infection. Scand J Infect Dis. 2007. 39: 272
8. Khurram M. Management of adult dengue shock syndrome patients not improving with DEAG guidelines based therapy. J Rawalpindi Med Coll. 2016. 20: 2-6
9. Kumar R, Prakash O, Sharma BS. Dengue hemorrhagic fever: A rare presentation as atypical acute subdural hematoma. Pediatr Neurosurg. 2008. 44: 490-2
10. Mahale RR, Mehta A, Shankar AK, Srinivasa R. Delayed subdural hematoma after recovery from dengue shock syndrome. J Neurosci Rural Pract. 2016. 7: 323-4
11. Maheshwari V, Kumar S, Kumar A, Kumar A. Spontaneous subdural hematoma of dorsal spine secondary to dengue fever: A rare case report with review of literature. Asian J Neurosurg. 2019. 14: 550-2
12. Mittal M, Jain N. Subdural haematoma and axonal polyneuropathy complicating dengue fever. BMJ Case Rep. 2011. 2011: bcr1220103672
13. Rothman AL, Ennis FA. Immunopathogenesis of Dengue hemorrhagic fever. Virology. 1999. 257: 1-6
14. Schaefer TJ PP, Wolford RW.editors. Dengue Fever. Treasure Island, FL: StatPearls Publishing; 2018. p.
15. Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, Raengsakulrach B. Neurological manifestations of dengue infection. Lancet. 2000. 355: 1053-9