- Department of Neurosurgery, Duke University School of Medicine, Baltimore, MD, United States.
- Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States.
- Department of Neurosurgery, Duke University Medical Center, Durham, North Carolina, United States.
- Department of Neurosurgery, Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, North Carolina, United States.
Correspondence Address:
Peter E. Fecci, MD, PhD, Professor of Neurosurgery, Preston Robert Tisch Brain Tumor Center, Department of Neurosurgery, Duke University Medical Center, Durham, NC, USA.
DOI:10.25259/SNI_1000_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Aden P. Haskell-Mendoza1, Ethan S. Srinivasan1,2, Alexander D. Suarez3, Peter E. Fecci4. Laser ablation of a sphenoid wing meningioma: A case report and review of the literature. 14-Apr-2023;14:138
How to cite this URL: Aden P. Haskell-Mendoza1, Ethan S. Srinivasan1,2, Alexander D. Suarez3, Peter E. Fecci4. Laser ablation of a sphenoid wing meningioma: A case report and review of the literature. 14-Apr-2023;14:138. Available from: https://surgicalneurologyint.com/surgicalint-articles/12256/
Abstract
Background: Meningiomas are the most common primary central nervous system neoplasm in the United States. While the majority of meningiomas are benign, the World Health Organization (WHO) Grade I tumors, a not-insignificant proportion of tumors are in anatomically complex locations or demonstrate more aggressive phenotypes, presenting a challenge for local disease control with surgery and radiation. Laser interstitial thermal therapy (LITT) consists of stereotactic delivery of laser light for tumor ablation and is minimally invasive, requiring implantation of a laser fiber through a cranial burr hole. Herein, we demonstrate the first use of this technology in a progressive atypical sphenoid wing meningioma for a previously resected and irradiated tumor.
Case Description: A 47-year-old female was diagnosed with a left-sided atypical meningioma, the WHO 2, of the sphenoid wing following acute worsening of bitemporal headache and dizziness. Given neurovascular involvement, a subtotal resection was performed, followed by stereotactic radiosurgery. Following progression 9 months from resection, the patient elected to proceed with LITT. The patient’s postoperative course was uncomplicated and she remains progression free at 24 months following LITT.
Conclusion: We present the first use of LITT for a sphenoid wing meningioma documented in the literature, which demonstrated enhanced disease control for a lesion that was refractory to both surgery and radiation. LITT could represent an additional option for local control of progressive meningiomas, even in locations that are challenging to access surgically. More evidence is needed regarding the technical nuances of LITT for lesions of the skull base.
Keywords: Brain tumors, Case report, Laser ablation, Laser interstitial thermal therapy, Skull base, Sphenoid wing meningioma
INTRODUCTION
Meningiomas are the most common primary neoplasm of the central nervous system (CNS) in the United States.[
In patients with recurrent high-grade meningioma, laser interstitial thermal therapy (LITT) may represent a minimally invasive alternative to open resection for eliciting surgical cytoreduction. Briefly, in the case of the NeuroBlate® system, a laser fiber is stereotactically placed within a target lesion using a standard burr hole, and 1064 nm Nd: YAG laser light is used to produce coagulative necrosis under real-time monitoring with magnetic resonance (MR) thermography. This mechanism has been adopted for ablation or cytoreduction in a variety of intracranial pathologies, including epileptic foci and movement disorders as well as both primary and recurrent gliomas and brain metastases. As the procedure is minimally-invasive, it may significantly reduce operative morbidity in previously-resected or irradiated recurrent disease.[
CASE DESCRIPTION
Patient presentation
A 47-year-old woman presented to urgent care with acute worsening of bitemporal headache and dizziness over the course of 2 weeks. She was noted to have had subjective personality changes in the previous several months, but otherwise her neurological examination was non-focal. Referral to an outside emergency department revealed a large left mass arising from the sphenoid wing measuring 5.2 × 4.2 × 6.1 cm with significant vasogenic edema on the frontotemporal regions, 1 cm of midline shift, and enhancing nodular and cystic components on MR imaging (MRI), with displacement of the middle cerebral artery (MCA) M1 and M2 segments and invasion of the calvarium. Initial imaging is shown in
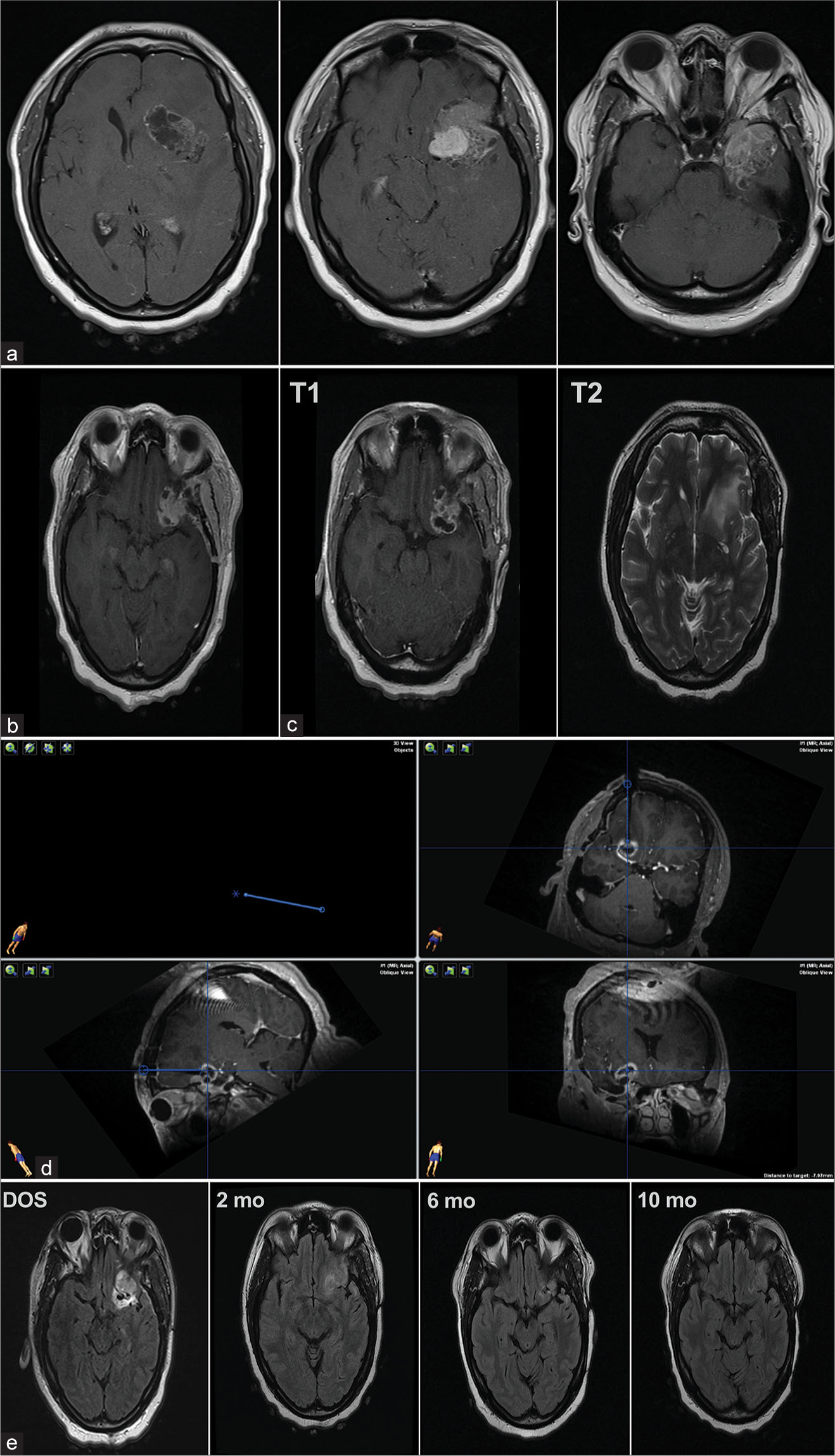
Figure 1:
Representative magnetic resonance imaging of the tumor at varying time points. (a) Contrasted T1 images before resection (b) Contrasted T1 image post-resection, pre- intensity-modulated radiation therapy (c) T1 (left) and T2 (right) images post-stereotactic radiosurgery, pre-laser interstitial thermal therapy (LITT). (d) The chosen supraorbital biopsy/LITT tract terminating anterior to the middle cerebral artery is shown (clockwise from top right) in axial, coronal, and sagittal views in neuronavigation software. (e) Post-LITT fluid attenuated inversion recovery imaging intraoperatively (day of surgery, DOS) and at 2 months (2 mo), 6 months (6 mo), and 10 months (10 mo), respectively.
Initial surgery
The patient had a preoperative cerebral angiogram with particle embolization of feeding vessels from the left middle meningeal artery. She then underwent a left frontotemporal craniotomy with osteotomy of the greater wing of the sphenoid. A subtotal Simpson grade IV resection was performed, limited by tumor encapsulation of the third nerve and involvement of the striate vessels of the inferior frontal lobe. Postoperatively, the patient had a new left upper eyelid ptosis and facial numbness at the craniotomy site, but was otherwise without complications. She was discharged home on postoperative day (POD) 2 without incident.
Pathology
Histopathological assessment revealed a spindle-cell neoplasm with whorling and collagen deposition. There was nuclear atypia and increased mitotic figures with some areas of necrosis, but no invasion of brain tissue. The KI-67 index was 10% and the lesion was epithelial membrane antigen positive and signal transducer and activator of transcription 6 negative. The final WHO integrated diagnosis was atypical meningioma, the WHO Grade 2.
Postoperative course
The patient developed significant left-sided headaches in the area of her craniotomy for which she began taking butalbital/ acetaminophen. Six-week postoperative MRI revealed 2.3 × 2.7 cm of residual enhancing tumor along the left sphenoid wing as expected [
LITT and post-LITT course
The patient was placed in the supine position. A supraorbital approach along the long axis of tumor was chosen for biopsy and laser ablation. The target location of the fiber tip was planned for the base of the frontal lobe in front of the MCA. Biopsy to rule out radiation necrosis or development of higher-grade features again demonstrated atypical meningioma, the WHO Grade 2. Following biopsy, the patient was transitioned into the intraoperative MRI and the lesion was ablated from deep to superficial with multiple rounds of lasing in 360° under real-time MR thermography monitoring. Special caution was taken to create a trajectory sparing the cranial nerves and other eloquent structures, as shown in neuronavigation software [
DISCUSSION
This case represents the first use of LITT in a patient with a progressive atypical sphenoid wing meningioma. Given the limited availability of high-quality evidence for imaging follow-up and reoperation for recurrent disease, management of recurrent meningiomas is complex.[
Thus far, LITT has been infrequently used in meningiomas. As 80% of meningiomas are benign, the WHO Grade 1 tumors that may be asymptomatic and simply followed on imaging, while convexity or parafalcine/parasagittal lesions are often definitively treated with open surgery alone, the majority of patients will not have progression such that LITT is indicated.[
In our review of the literature, we were unable to identify any articles discussing the use of LITT in skull-base meningiomas. In 2018, meningiomas made up only 4% of the LAANTERN database, which includes data on patients treated with LITT through the NeuroBlate system.[
CONCLUSION
In our patient’s case, LITT appeared to halt further progression despite the worrisome lack of response to SRS. The procedure was performed safely and without complication, with stable follow-up imaging out to 10 months. As the first known example of LITT for progressive atypical skull-base meningioma, this report demonstrates feasibility and potential benefit of the application. In the future, LITT could be employed more frequently in appropriately selected meningiomas of the skull base when delicate vasculature and cranial nerves can be avoided. As the applications of LITT continue to increase in neurosurgical oncology, discussion of novel use cases provides significant value in guiding evolution of the technology.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Peter E. Fecci is a consultant for Monteris Medical.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Agha RA, Franchi T, Sohrabi C, Mathew G, Kerwan A. The SCARE 2020 guideline: Updating consensus surgical case report (SCARE) guidelines. Int J Surg. 2020. 84: 226-30
2. Brastianos PK, Galanis E, Butowski N, Chan JW, Dunn IF, Goldbrunner R. Advances in multidisciplinary therapy for meningiomas. Neuro Oncol. 2019. 21: i18-31
3. Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol. 2018. 14: 2161-77
4. de Groot JF, Kim AH, Prabhu S, Rao G, Laxton AW, Fecci PE. Efficacy of laser interstitial thermal therapy (LITT) for newly diagnosed and recurrent IDH wild-type glioblastoma. Neurooncol Adv. 2022. 4: vdac040
5. Eichberg DG, Casabella AM, Menaker SA, Shah AH, Komotar RJ. Parasagittal and parafalcine meningiomas: Integral strategy for optimizing safety and retrospective review of a single surgeon series. Br J Neurosurg. 2020. 34: 559-64
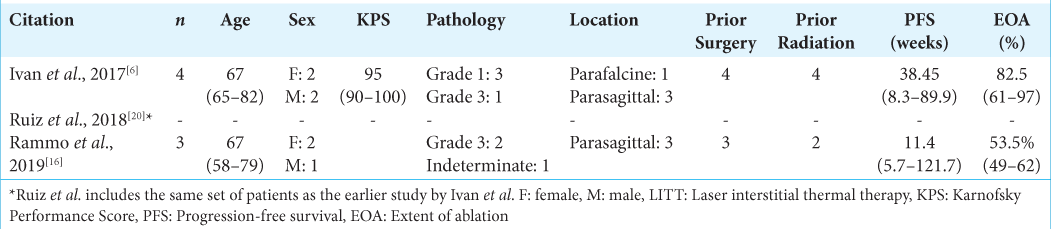
6. Ivan ME, Diaz RJ, Berger MH, Basil GW, Osiason DA, Plate T. Magnetic resonance-guided laser ablation for the treatment of recurrent dural-based lesions: A series of five cases. World Neurosurg. 2017. 98: 162-70
7. Jermakowicz WJ, Cajigas I, Dan L, Guerra S, Sur S, D’Haese PF. Ablation dynamics during laser interstitial thermal therapy for mesiotemporal epilepsy. PLoS One. 2018. 13: e0199190
8. Johnson RA, Do TH, Palzer EF, Cramer SW, Hanson JT, Huling JD. Pattern of technology diffusion in the adoption of stereotactic laser interstitial thermal therapy (LITT) in neuro-oncology. J Neurooncol. 2021. 153: 417-24
9. Kato A, Fujimoto Y, Hashimoto N, Taniguchi M, Kinoshita M, Hirayama A. Radiofrequency thermal ablation for recurrent meningioma extending extracranially. Acta Neurochir (Wien). 2005. 147: 543-50 discussion 550
10. Kato A, Fujimoto Y, Taniguchi M, Hashimoto N, Hirayama A, Kinoshita M. Volumetric thermal devascularization of large meningiomas. J Neurosurg. 2004. 101: 779-86
11. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
12. Magill ST, Lee DS, Yen AJ, Lucas CH, Raleigh DR, Aghi MK. Surgical outcomes after reoperation for recurrent skull base meningiomas. J Neurosurg. 2019. 130: 876-83
13. Nassiri F, Liu J, Patil V, Mamatjan Y, Wang JZ, Hugh-White R. A clinically applicable integrative molecular classification of meningiomas. Nature. 2021. 597: 119-25
14. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021. 23: iii1-105
15. Pisipati S, Smith KA, Shah K, Ebersole K, Chamoun RB, Camarata PJ. Intracerebral laser interstitial thermal therapy followed by tumor resection to minimize cerebral edema. Neurosurg Focus. 2016. 41: E13
16. Rammo R, Scarpace L, Nagaraja T, Lee I. MR-guided laser interstitial thermal therapy in the treatment of recurrent intracranial meningiomas. Lasers Surg Med. 2019. 51: 245-50
17. Rennert RC, Khan U, Tatter SB, Field M, Toyota B, Fecci PE. Patterns of clinical use of stereotactic laser ablation: Analysis of a multicenter prospective registry. World Neurosurg. 2018. 116: e566-70
18. Rieke V, Pauly KB. MR thermometry. J Magn Reson Imaging. 2008. 27: 376-90
19. Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015. 122: 4-23
20. Ruiz A, Diaz RJ, Buttrick S, Ivan M, Desai M, Komotar RJ. Preliminary experience on laser interstitial thermal ablation therapy in the treatment of extra-axial masses: Indications, imaging characterization and outcomes. Cureus. 2018. 10: e2894-94
21. Srinivasan ES, Grabowski MM, Nahed BV, Barnett GH, Fecci PE. Laser interstitial thermal therapy for brain metastases. Neuro Oncol Adv. 2021. 3: v16-25
22. Wright J, Chugh J, Wright CH, Alonso F, Hdeib A, Gittleman H. Laser interstitial thermal therapy followed by minimal-access transsulcal resection for the treatment of large and difficult to access brain tumors. Neurosurg Focus. 2016. 41: E14