- Professor of Neurosurgery, Sina Hospital, Tehran, Iran
- Professor of Neurosurgery, Arad Hospital, Tehran, Iran
- Assistant Professor of Neurosurgery, Sari University of Medical Sciences, Sari, Iran
- Department of Epidemiology, Head Department of Health in Emergencies and Disasters, School of Public Health and Institute of Public Health Research, Tehran University of Medical Sciences, Tehran, Iran
- Assistant Professor of Neurosurgery, Firoosgar Hospital, Iran University of Medical Sciences, Iran
Correspondence Address:
Abbas Amirjamshidi
Assistant Professor of Neurosurgery, Firoosgar Hospital, Iran University of Medical Sciences, Iran
DOI:10.4103/2152-7806.157074
Copyright: © 2015 Amirjamshidi A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Amirjamshidi A, Abbasioun K, Amiri RS, Ardalan A, Ramak Hashemi SM. Lateral orbitotomy approach for removing hyperostosing en plaque sphenoid wing meningiomas. Description of surgical strategy and analysis of findings in a series of 88 patients with long-term follow up. Surg Neurol Int 14-May-2015;6:79
How to cite this URL: Amirjamshidi A, Abbasioun K, Amiri RS, Ardalan A, Ramak Hashemi SM. Lateral orbitotomy approach for removing hyperostosing en plaque sphenoid wing meningiomas. Description of surgical strategy and analysis of findings in a series of 88 patients with long-term follow up. Surg Neurol Int 14-May-2015;6:79. Available from: http://surgicalneurologyint.com/surgicalint_articles/lateral-orbitotomy-approach-removing-hyperostosing-en/
Abstract
Background:Sphenoid wing meningiomas extending to the orbit (ePMSW) are currently removed through several transcranial approaches. Presenting the largest surgical cohort of hyperostosing ePMSW with the longest follow up period, we will provide data supporting minilateral orbitotomy with excellent exposure for wide resection of all compartments of the tumor.
Methods:A retrospective survival analysis is made of the data cumulated prospectively during a period of 34 years, including 88 cases of ePMSW with a mean follow up period of 136.4 months. The impact of preoperative variables upon different outcome measures is evaluated. Standard pterional craniotomy was performed in 12 patients (C) while the other 76 cases underwent the proposed modified lateral miniorbitotomy (LO).
Results:There were 31 men and 57 women. The age range varied between 12 and 70 years. Patients presented with unilateral exophthalmos (Uex) ranging between 3 and 16 mm. Duration of proptosis before operation varied between 6 months and 16 years. The status of visual acuity (VA) prior to operation was: no light perception (NLP) in 16, light perception (LP) up to 0.2 in 3, 0.3–0.5 in 22, 0.6–0.9 in 24, and full vision in 23 patients. Postoperatively, acceptable cosmetic appearance of the eyes was seen in 38 cases and in 46 mild inequality of P = 0.05). The major determinant of tumor regrowth was the technique of LO (P = 0.008).
Conclusion:Using LO technique, the risky corners involved by the tumor is visualized from the latero-inferior side rather than from the latero-superior avenue. This is the crucial milestone to achieve aggressive removal of all the involved compartments of the lesion. Satisfactory cosmetic result is reported using mini LO technique after widely exposing and removing the hyperostotic bone down to the subtemporal fossa with only simple repair of the dura without cranioplasty.
Keywords: Lateral orbitotomy, meningioma, orbital tumors, proptosis, sphenoid wing meningioma, spheno-orbital meningioma, unilateral exophthalmos.
INTRODUCTION
Sphenoid wing (SW) en plaque meningioma (ePM) is a subgroup of meningiomas defined by its specific character presenting with a rather thin sheath of soft tumor tissue accompanied by disproportionate and extensive bone hyperostosis. ePMs may occur anywhere along the central nervous system (CNS).[
In this retrospective analysis of a prospective acquisition of database including 88 consecutive cases of what is called ePM of the sphenoid wing (ePMSW), we will present and discuss: (i) The reasons for changing our attitude in surgical technique, (ii) analyze the impact of variants upon the long-term follow up results of the patients with the end point of focal tumor recurrence, and (iii) it will be hypothesized that the term of ‘ePMSW’ should only be specified for a single group of ‘ePM’ of the SW with specific characteristics.
CLINICAL MATERIAL AND METHODS
Inclusion criteria and patient population
Among all the patients undergoing surgical intervention with the diagnosis of unilateral exophthalmos (Uex) in the section of skull base surgery (451 cases) there were 88 patients who fulfilled the criteria to be included in this study. Close cooperation between different specialties joined in our skull base interdisciplinary team, which included neurosurgeons, neurologist, neuro-ophthalmologist, neuro-otologist, neuro-radiologist, and neuro-pathologist, and was the main reason that made this study a reasonable and watchful bench.
We included all the patients admitted with the diagnosis of Uex whose histopathology report was meningioma with involvement of the sphenoid bone. There were two cases with multiple meningiomas/meningiomatosis and were excluded from this series.
The selection criteria were:
Hyperostosis of the greater/lesser wing of the sphenoid bone. This usually involved the pterional synostosis, the lesser and the greater SWs above and below the superior orbital fissure (SOF) and could involve the elements of the ipsilateral clinoid process and optic foramen Enhancement of the soft tissue of the tumor is visible in postcontrast enhancing T1W magnetic resonance imaging (MRI), located mainly along the SW and around the orbital fissure. The soft tissue tumor could be visible within the cone and lateral compartment of the orbit and also extend underneath the temporalis muscle extracranially. Extension of tumor tail could also exist along the ipsilateral clinoid, in the subfrontal region over the roof of the orbit, toward the cavernous sinus and its lateral wall, enhancing the wall of the sphenoid or ethmoid sinuses, and ensheathing the dura of the subtemporal fossa extending toward the petrous surface If there were no hyperostosis of the elements of the SW, or the tumor mass was mainly located around the anterior clinoid process (ACP) or enrooted from within the cavernous sinus or was mainly a variant of optic nerve sheath tumor or was a pure meningioma of the lateral/mid 3rd of the SW, they were excluded.[
The study period was 34 years, from June 1979 to June 2013.
Neurological and ophthalmological examination
All patients underwent complete pre- and postoperative neurological examinations and signs and symptoms denoting the duration of illness were recorded. Visual acuity (VA) (Snellen notation), visual field (VF) (Goldmann or Humphrey perimetery), and funduscopy were all performed by the neuro-ophthalmologist.
Imaging evaluations
The preoperative neuroradiological evaluation was performed in all cases. MRI, computed tomography (CT) and 3-dimensional reconstruction CT scan (3DRCTS) were all reported by the neuro-radiologist.
Infiltration and enhancement of the periorbita, dura mater of the temporal fossa, cavernous sinus, and the extra-calvarial soft tissue were assessed on postcontrast MR images, while osseous extension was assessed on CT scans before and after contrast enhancement.
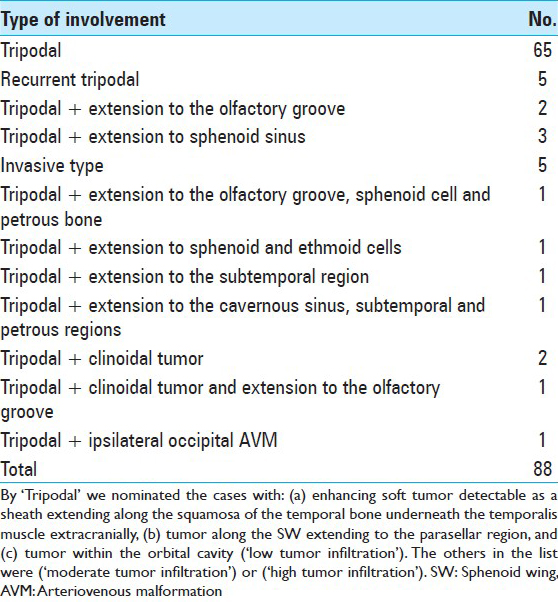
All of the ePMs in this series presented with the four radiological characteristics defined as: (a) Hyperostosis of the wings of the sphenoid, (b) enhancement of the soft tissue of the periorbita (pod 1), (c) variable enhancement of the dura of the temporal fossa (pod 2), and (d) enhancement of the soft tissue beneath the temporalis muscle highly suspicious for extra-calvarial involvement of the tumor (pod 3). According to the above findings, the nomination of ‘tripodal meningioma’ will be appropriated. Based on the different degrees of involvement of other compartments besides the above-mentioned structures, we selected the term of ‘radiologically invasive ePM’ (RIePM) for those cases [
The cases with only tripodal enhancement in MRI were designated as ‘low tumor infiltration.’ Those with extension to the subfrontal and olfactory groove and along the cavernous sinus were designated as ‘moderate tumor infiltration’ and those with tumor extension or tail to the paranasal and sphenoid sinuses, to the extracranial parapharyngeal and subtemporal regions, crossing the midline in the anterior fossa are coined as ‘high tumor infiltration’ [
Clinical and radiological follow up
It was presumed that all patients undergo clinical follow-up with complete visual and neurological examinations and cerebral MR/CT imaging on a regular basis every 6–12 months after primary intervention. To analyze the extent of surgical tumor removal, operative notes were reevaluated regarding the amount of bone removal and resection of intradural or intraorbital tumors. The first and last clinical examination and imaging taken in the follow-up period were compared in this study and the clinical and radiologic findings were measured numerically and by observation of the variables [
Surgical intervention
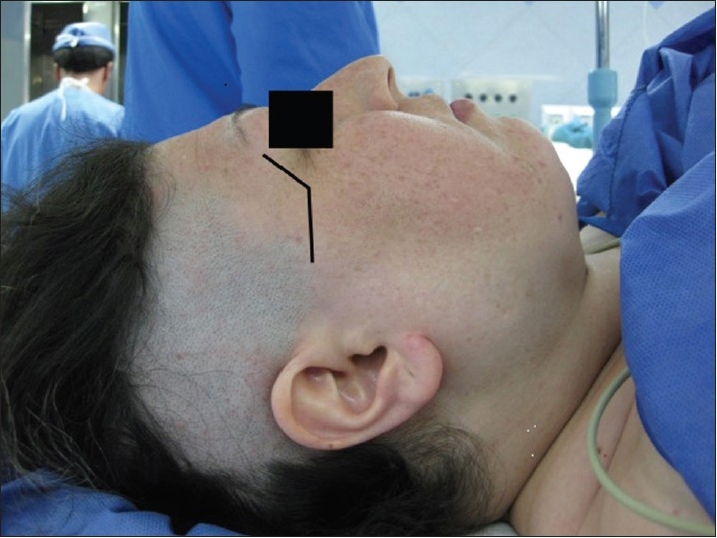
The patient's head is fixed in a 3-pin Mayfield head holder and is rotated 30° to 45o to the contralateral side. Brain relaxation and control of intraglobal pressure was maintained using dehydrating agents and moderate hyperventilation prior to and during surgery. A S-shaped skin incision is made starting from within the eyebrow, along the lateral 3rd and down to the extreme lateral end of the lateral canthus, and then turned horizontally along the zygomatic arch to a finger width distance in front of the tragus to prevent any damage to the frontal branch of the facial nerve [
Figure 2
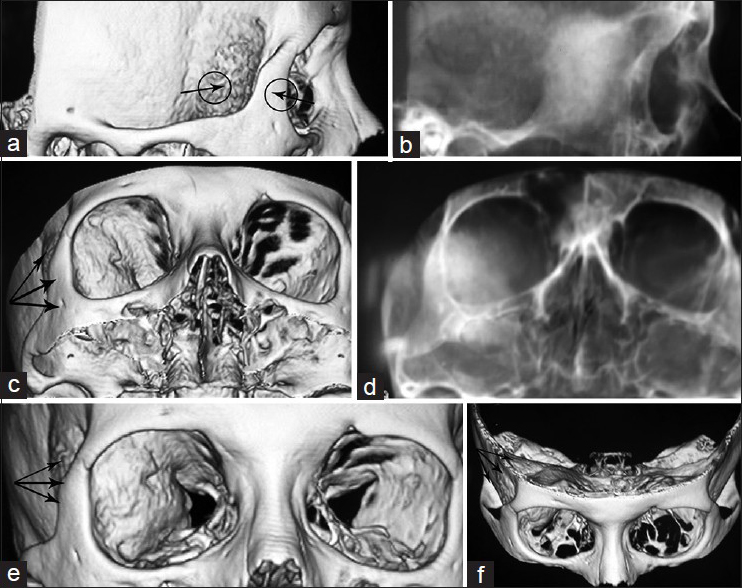
Three dimensional bone formatted CTS of the skull along with the skull X-ray adjusted for each, defining the gates through which the hyperostotic bone is visualized (a and b black arrows in the circles) and the avenues through which the instruments can better reach the depth to remove decompress the involved/hyperostotic bone (black triple arrows in c, d and e), and the black triple arrows when approaching via standard/pterional craniotomy (f) the view when is operated through the traditional pterional approach
Figure 3
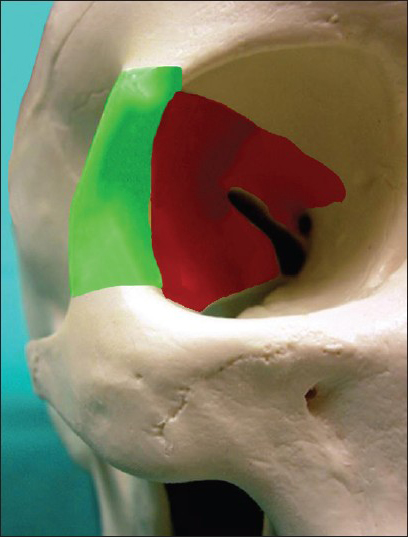
The schematic drawing showing the rim of the orbit removed and refixed at the end (green shaded) and the segments of the hyperostotic bone, which is necessary to be drilled out in most of the cases to achieve complete excision of the possibly involved bony compartments around the SOF down to the inferior orbital fissure and to decompress the optic canal from the lateral side (red shaded)
The intradural component of the tumor can be removed solely by cutting the dura along the normal looking boundary of the tumor in all directions. Two points should be highlighted in this stage: (i) Delicate dissection of dura should be done around the SOF with careful coagulation of the dural folds infiltrated by the tumor to decrease the amount of residual tumor cells in the vicinity of the orbital fissure and (ii) the entire tumoral infiltration of the dura at the base of frontal lobe and over the roof of the orbit, the involved dura of the temporal fossa in its anterior, inferior, and medial or internal part located along the lateral wall of the cavernous sinus should be either removed or curetted and coagulated.
The orbital compartment of the tumor can either be seen or palpated at this stage. Extension of the tumor to the roof, along the most inferior part and toward the cone of the orbit, can be dissected and removed with this exposure. Reduction of the tumor mass can be achieved using either piece meal technique or using ultrasonic aspirator. It is better to leave tumor spicules behind especially in the cone of the orbit where the elements entering the orbit via SOF might be infiltrated by the tumor cells.
Dural repair can be done with a large patch of pericranial graft removed from the temporo-parietal region and sutured only in some points, and covered with Gelfoam or Surgicel. Neither the roof, the lateral wall of the orbit nor the defect of the temporal bone was reconstructed in any of our cases. The bone strut removed from the orbito-zygomatic arch is replaced and fixed in an appropriate and feasible way and periosteum sutured over it. Vacuum drain with moderate negative pressure is placed underneath the temporalis muscle for 48 h. Skin is sutured in subcuticular manner. Lumbar puncture is performed once or twice during the postoperative period to manage subgaleal cerebrospinal fluid (CSF) collection.
Illustrative cases
Case 1
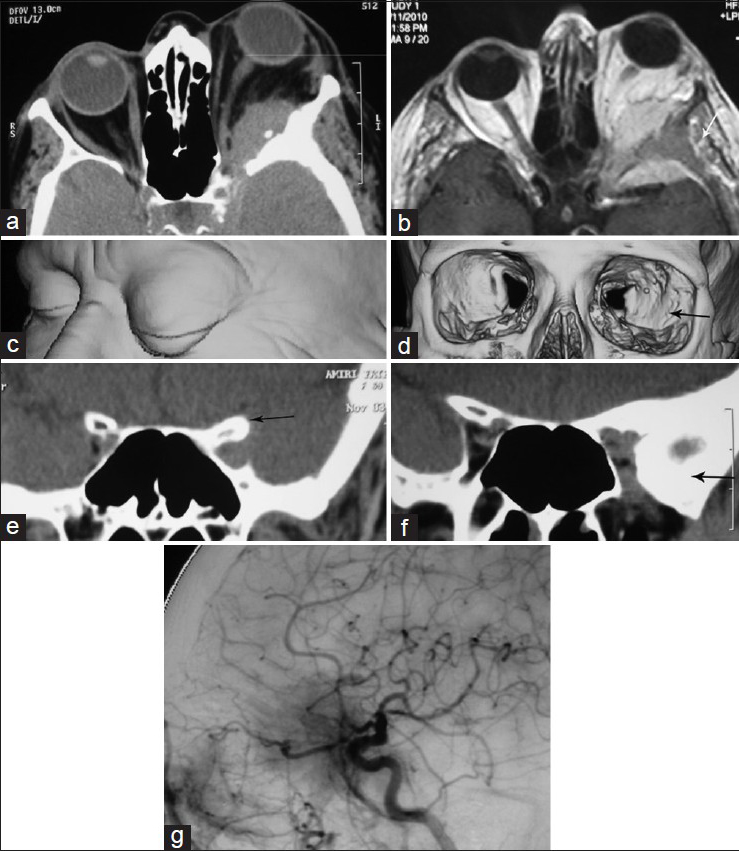
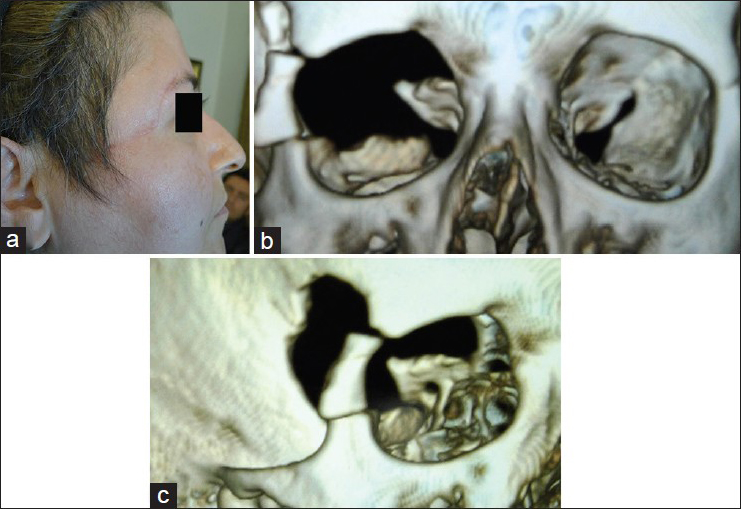
A 69-year-old female was admitted with progressive left Uex of more than 6 years duration. The exophthalmos (Ex) was 7 mm with inwards and downwards displacement. It was irreducible and nonpainful without any extraocular muscle palsy (EOMP). VA was 0.4 on Snellen notation with some restriction of VF on Humphrey perimeter. In fundoscopy, optic disc was blurred and whitish discolored with no optocilliary shunt. CT scan with 3D reconstruction identified hyperostotic greater and lesser SWs and part of the ACP in different views. The soft tumor tissue extended in a carpet fashion in the temporal fossa with a maximum thickness of 4–5 mm to the cavernous sinus but it did not infiltrate it. The intraorbital extension of the tumor was voluminous, compressing the optic nerve both within the optic foramen and the cone of the orbit. Weekly enhancing tumoral tissue was also present underneath the temporalis muscle. Another very thin enhancing carpet of tumor was also seen hanging from the lateral roof of the sphenoid sinus, which could be either tumor extension or congested mucosal coverage. Angiography performed in another center revealed moderate tumor blush fed through the branches of the external and internal carotid arteries [
Figure 4
(a and b) contrast enhanced CTS and MRI showing the intraorbital, temporal intra- and extra-cranial (small white arrow) of the tumor, c and d) three dimensional imaging of the face demonstrating the pattern of EX in (c), and bony cavity of the orbits showing asymmetric orbital fissures and squeezing of the left orbital fissure by the hyperostotic lesser and greater wings of the sphenoid bone (black arrow) in (d). (e) is the coronal view mainly illustrating the hperostotic ACP while (f) demonstrates hyperostosis of lesser and greater wings of the sphenoid bone and asymmetric orbital fissures. (g) is the lateral view of DSA showing the tumor blush located in the anterior temporal region. It is mainly fed through the meningeal branches of the ascending pharyngeal artery and the cavernous branches of the internal carotid artery and recurrent meningeal branches of the ophthalmic artery
Case 2
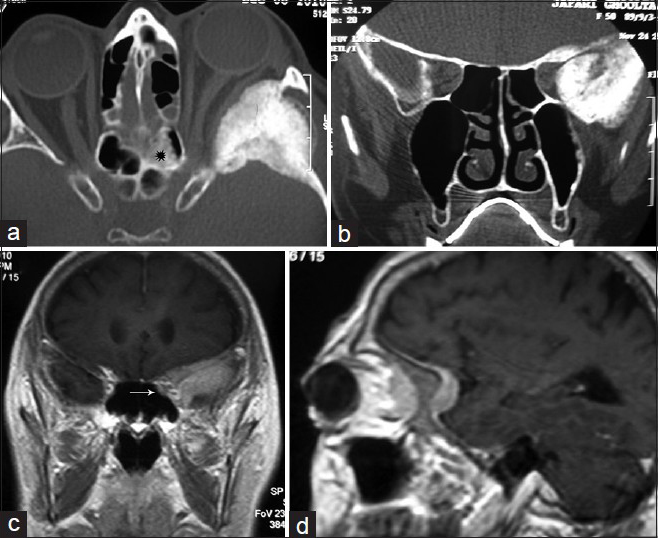
This 56-year-old female was admitted with UeX of 4 years duration and VA of 0.6. Imaging showed remarkable hyperostosis of all the SW, normal looking ACP, and thin layer of enhancing soft tumoral tissue encasing the hyperostotic area. A very thin layer of soft tissue thickening was visible both in CT scan and MRI just in the vicinity of the mucosal layer of the sphenoid sinus [
Figure 5
(a and b) horizontal and coronal views of CTS hyperostotic wings of the SW and temporal squamosal plate and asymmetric orbital fissures squeezed in the left side without affection of ACP, (c and d) contrast-enhanced MRI elucidating a thin layer of tumoral tissue enhancement along the SW and subfrontal dura, underneath the temporalis muscle and highly suspicious for extending into the orbit. There is a bright sclerotic thickening within the posterior ethmoid sinus in the left side (black star in a) and an enhancing soft tissue nodule located in rather the same area in the ethmoid region visible in MRI (c, white arrow)
Case 3
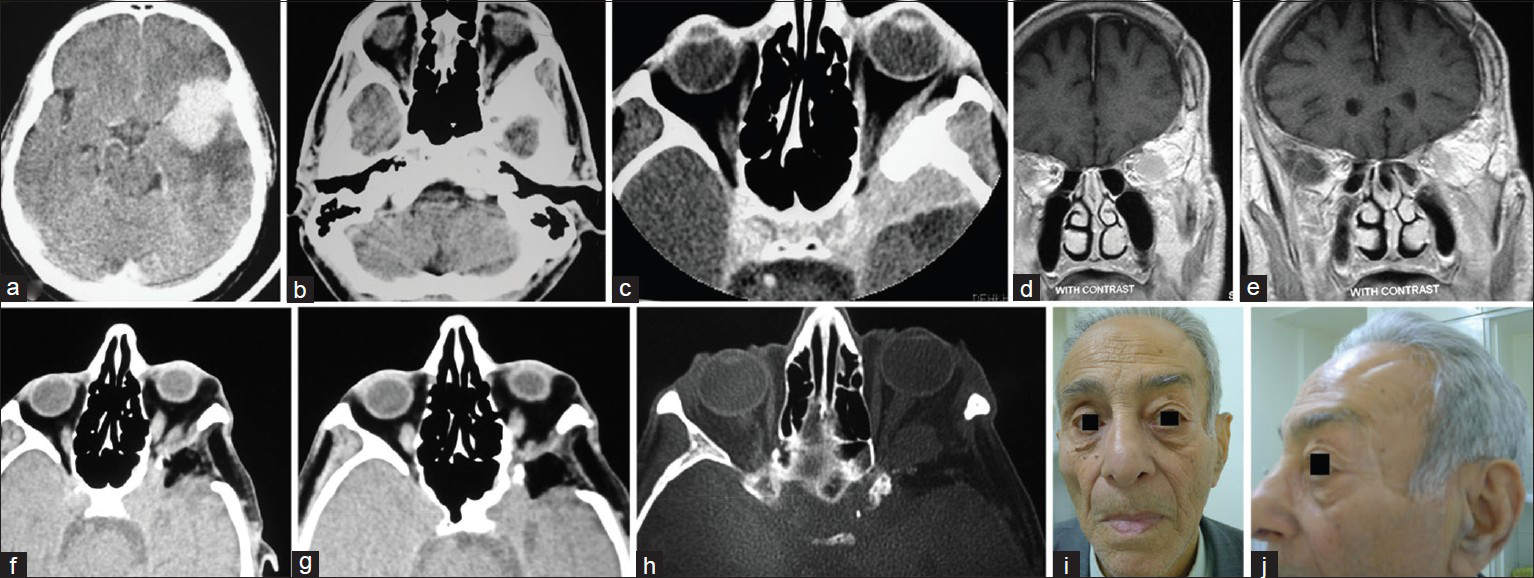
An 81-year-old male was admitted on June 2004 with left Uex. In May 1994 he underwent craniotomy in another center for SOM presenting with Uex and decreased vision secondary to a tumor mass arising from the left SW with hyperostosis visible in contrast enhanced CT scans. He did well after the first operation but vision decreased and Ex reappeared after 6 years. New imaging revealed hyperostosis of the SW and enhancing tumor mass within the lateral compartment of the orbit extending along the wall of the cavernous sinus to the temporal tip, and beneath the temporalis muscle [
Figure 6
(a and b) contrast-enhanced CTS available from the first admission showing the hyperostotic SW the tumor mass in the temporal and subfrontal regions before craniotomy, (c) contrast enhanced CTS in the 2nd admission showing the remainder of the hyperostotic ridge, enhancing tumor and persisting EX, (d and e) contrast-enhancing T1W coronal MRI in the 2nd admission demonstrating the site of previous craniotomy and bulging SW involved by the tumor recurrence, (f and g) CTS performed after second intervention via the lateral approach showing appropriate bony decompression of the orbit and regression of EX, (h) MRI confirming tumor remnant within the cone of the orbit, and (i and j) face of the patient after long follow-up with good cosmetic features and no atrophy of the temporalis muscle
RESULTS
Before operation
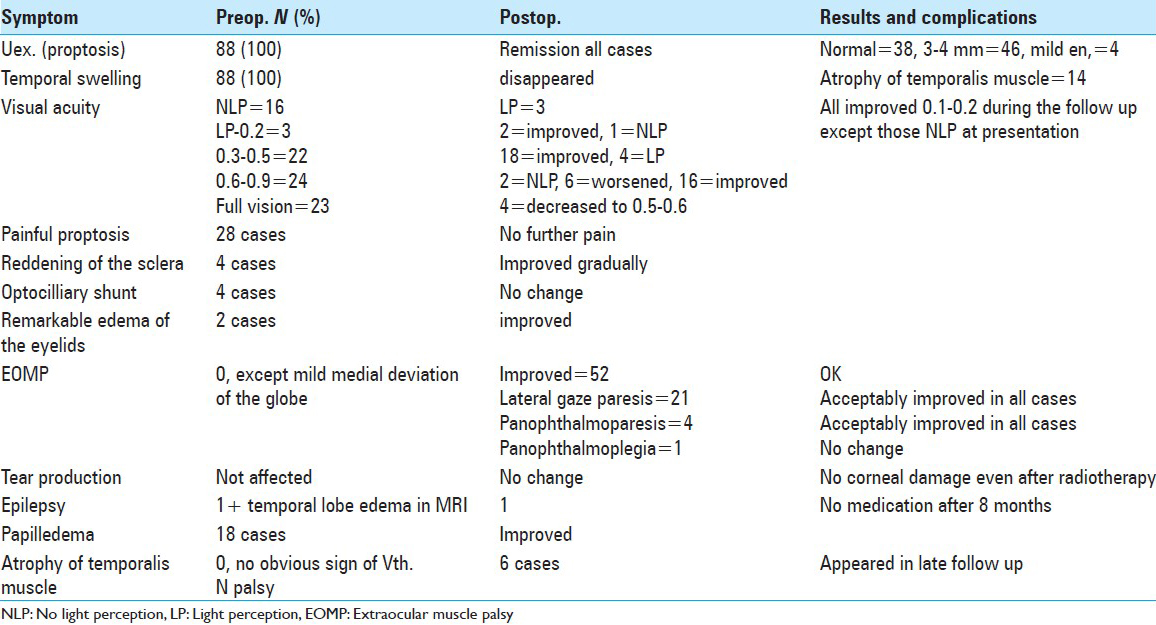
There were 88 cases including 31 men and 57 women (M/F ratio of 1:1.8). The symptoms at presentation are summarized in
Outcome
Mild En occurred in the few postoperative days and usually disappeared after removing the surgical drain. Regarding remission of Uex, the difference between the two eyes up to 1 mm was considered as ‘acceptable remission.’ There were 38 cases with acceptable cosmetic appearance of the eyes regarding the location of the globes, 46 cases with mild inequality of <2mm and 4 with mild En 2–4 mm when visited for the first time almost 3 months following operation. This remained unchanged to their last visit. Previously noted swelling of the temporalis muscle was not detectable any more. Three of the patients who were blind preoperatively gained LP during follow up visits. Among those who had a poor quality of vision, two improved and one became almost blind after operation with no further improvement. All the cases with VA in the range of 0.3–0.5 improved after operation. Among those with good VA (0.5 to full vision), 2 became blind, vision decreased in 10, and VA improved or remained unchanged in the other 35 cases [
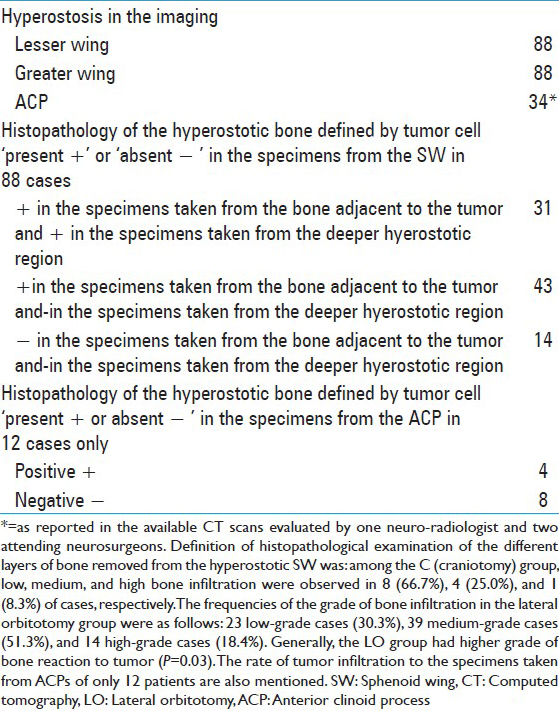
On histopathological examination, the sphenoid bone was infiltrated by meningioma in 53 (60.3%) of the cases. There has been no early reoperation needed in this series. Tumor recurrence occurred in 12 cases but reoperation was needed in 2 cases.
Radiotherapy
Focal radiation was delivered in the cases with (a) visible tumor residue or regrowth in follow-up imaging, (b) those with bone infiltration with tumor cells in pathological studies, and (c) patients with clinically evident Uex in the postop period (31 cases). No patient developed radiation-induced retinopathy or pituitary insufficiency.
Statistical analysis
For statistical analysis, SPSS v 16.0 was used. To describe the study variables, descriptive statistical measures were calculated including frequency, percentile, mean, and standard deviation whenever appropriate. Independent t-test was applied to compare the mean of continues variables between the two study groups. The association between two categorical variables was assessed using a Chi-square test. Whenever one or two of the study variables was ordinal in scale, the Chi-square for trend was applied. A multiple logistic regression was used to evaluate the determinants of the tumor recurrence as an outcome variable. For other two outcome variables, that is, improvement/cure of the Uex and improvement of VA, the multiple linear regression models was applied to assess the determinant factors. The P value less than 0.05 (P < 0.05) was considered statistically significant.
Output of the analysis
This 34-year follow-up study includes findings related to 88 cases of ePM including 12 cases (13.6%) who underwent craniotomy (C) and 76 cases (86.4%) who underwent lateral miniorbitotomy (LO) approach. Radiotherapy was delivered to 8 cases (66.7%) in group C and to 23 cases (30.3%) in group LO. The mean follow-up period was 179 months (±123.6) in group C, and 129.6 months (±103.0) in group LO.
Tables
Demographic variables
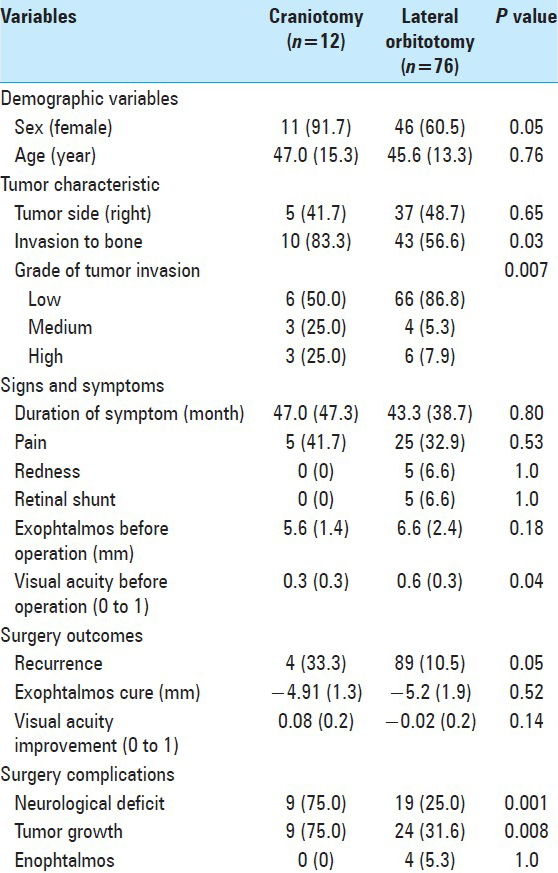
Eleven (91.7%) patients in group C and 46 patients (60.5%) in group LO were female (P = 0.05). Mean age of the cases in C and LO groups were 47.0 years (±15.3) and 45.6 (±13.3), respectively (P = 0.76).
Tumor characteristics
Five cases (41.7%) in group C and 37 cases (48.7%) in group LO had tumor in their right orbit (P = 0.65). Invasion to bone was observed in 10 cases (83.3%) of group C and 43 cases (56.6%) of group LO (P = 0.11). Among group C, low, medium, and high bone infiltration were observed in 6 (50.0%), 3 (25.0%), and 3 (25.0%) of cases, respectively. The frequencies of the grade of tumor invasion in the LO group were as follow: 66 low-grade cases (86.8%), 4 medium-grade cases (5.3%), and 6 high-grade cases (7.9%) [
Signs and symptoms
Duration of the symptoms prior to operation was 47.0 months (±47.3) in group C and 43.3 months (±38.7) in group LO (P = 0.80). Five patients (41.7%) in group C had pain, while that was experienced by 25 cases (32.9%) in group LO (P = 0.53). Five patients (6.6%) in group LO had eye redness and retinal shunt. None of these symptoms were observed in patients of the group C.
The mean of the amount of Uex before operation was 5.6 mm (±1.4) in group C and 6.6 mm (±2.4) in group LO (P = 0.18). The mean of VA before the operation was observed as 0.3 (±0.3) in group C, while this was 0.6 (±0.3) in group LO (P = 0.04). Even though the differences between some of the variants were significant in this analysis but considering the design of our study, that is, changing the technique from C to LO and achieving more experience steadily, the clinical interpretation of the differences could be debated.
Outcome of surgery
The tumor recurrence was observed in four cases (33.3%) of the group C, and eight cases (10.5%) in group LO (P = 0.05). The matter of increased ‘expertise of the surgeon’ could not be evaluated in this study because the follow up periods have not been equal between the more recent cases and those operated during the previous 5-year interval. The difference between Uex after operation compared to Uex prior to operation was 4.91 mm (±1.3) in group C and -5.2 mm (±1.9) in the group LO (P = 0.52). The degree of improvement in VA noted after operation was 0.08 (±0.2) in group C and -0.02 (±0.2) in the group LO (P = 0.14).
Complications of surgery
The frequency of neurological deficit was lower in group LO than the group C, that is, 25.0% vs. 75.0% (P = 0.001). Tumor growth was also less frequent in the group LO compared with the group C (31.6% vs. 75.0%) (P = 0.008). Enophthalmos (En) was noted in four cases (5.3%) who had LO surgery, while this problem was not observed in any case of group C.
Tumor growth after the operation correlated positively with tumor invasion to bone (P < 0.001), as it occurred in 50.9% and 17.1% of cases with and without tumor invasion to bone according to the histopathological reports, respectively. It occurred in 88.9% of high-grade, 71.4% of medium-grade, and only 27.8% of low-grade bone infiltrations [
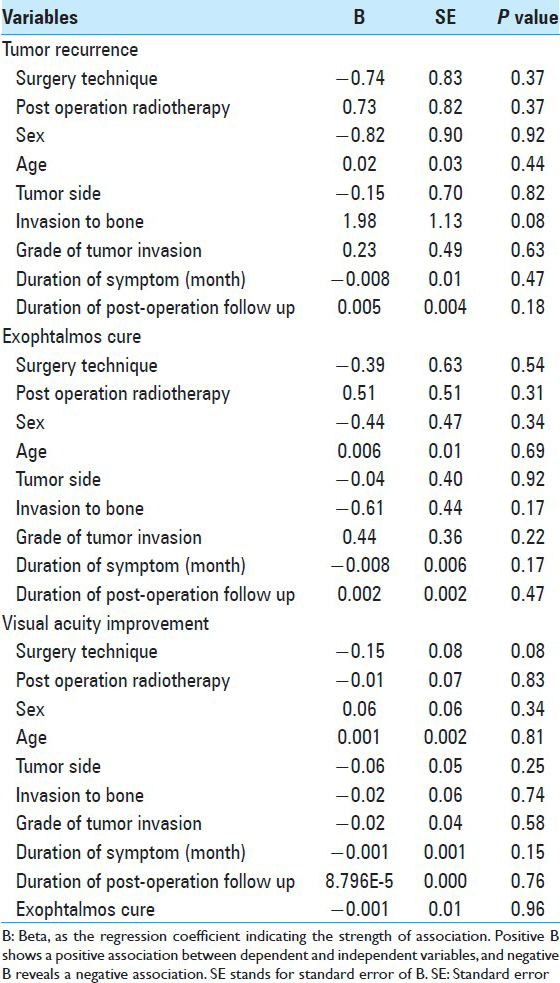
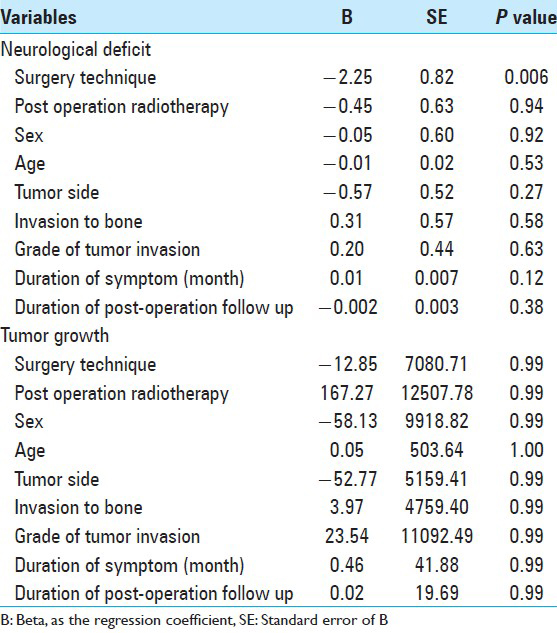
Results of the multiple regression analyses
DISCUSSION
Epidemiology
Sex distribution in our series was 31 men and 57 women (M/F ratio of 1/1.8), which are remarkably different from other reports showing ratios between 1/3 and 1/15 with an average of 1/3 to 5.[
The cause of hyperostosis
Hyperostosis frontalis interna, osteoma, focal erythroid hyperplasia, fibrous dysplasia, osteoblastic metastasis to the SW, sarcoidosis, and Paget disease are the main pathologies leading to hyperostosis in this region.[
Imaging evaluation
Extensive neuroimaging assessments are necessary including: (a) High-resolution CT scans with contrast material injection to evaluate bone as well as soft tissue involvement. Thin-cut coronal bone CT scan were taken with 3-dimensional reconstruction (3D recon) not only to detect even a small amount of hyperostosis but also to simplify surgical planning, (b) good resolution MRI with gadolinium enhancement done with fat-suppression and other possible sequences to detect any soft tissue tumor infiltration. Different authors have classified hyperostosis of SWMs into four types: Homogeneous, periosteal, layered, and diploic.[
Symptoms and their oncologic pathogenesis
The most common clinical presentation of ePMSW is Uex. Their Ex is usually slowly progressing, nonpulsating, irreducible, and painless. Hyperostosis of the elements of the SW, invasion of the tumor to the periorbita, intraorbital tumor extension, and venous stasis of both superior and inferior orbital veins are the main causes of these symptoms. Decreased vision, diplopia, ptosis, headache, and seizure may develop later in the course of the illness.[
All the tumors in our series were of meningotheliomatous type, WFNS grade I. Consequently, there were no potential relationship between histological features of these tumors and hyperostosis. We have only recently started to reexamine the specimens for immuno-histochemical staining and other growth labeling indices.
Operative approach
Decompression of the SOF is important for correcting the symptoms of Uex in all such cases. In the cases with visual impairment, it is strongly recommended to decompress the optic canal too.[
Historical background and comparison of technical points
In our communication, we present a series of 88 patients harboring SW meningioma with extension into the lateral or superolateral orbital compartments. Seventy-six underwent tumor resection via LO approach coupled with extensive resection of all components of the hyperostotic SW, thus avoiding formal craniotomy.
In 1889, Kronlein first proposed a lateral approach to the orbit, and in 1953 he modified the lateral incision from a “horseshoe” osteoplastic type to a transverse one extending posteriorly from the lateral canthus for 30–35 mm. There have been some other modifications in these techniques.[
Even though complete bony decompression is achievable in this way and excision of the soft tumor tissue looks to be more precise using microdissection, (near to Simpson grade I in the tripodal types in
Reconstruction technique and cosmesis
Depending on the extent of resection of the wall and roof of the orbit, most authors who performed these variants of craniotomy procedures recommend firm reconstruction of the orbital walls to avoid pulsating En and diplopia.[
By mini-approach, we do not intend to apply the term of “minimally invasive” technique. Removing as much bone to change the aspects of an ePMSW to a convexity meningioma, via the incision placed partly in the face is better not to be coined as “minimally invasive.”
Repairing the dura mater by autologous fascia, fat, and Gelfoam or Surgicel was sufficient in all of our cases and no disfiguring or pulsating En, fistula or meningocele were encountered in long-term follow up.
The issue of severe atrophy of the temporalis muscle occurred in 4/76 cases with LO mini-approach but 2/12 with C approach [
It looks disturbing that all or part of the scar is visible on the face and is not covered by hair. Half of the incision used for this approach is located within the eyebrow and the other half (at most 3–3.5 cm) is located along the maxilla, which is best be sutured in a subcuticular manner.
Role of adjuvant radiotherapy
Thirty-two of the cases in this series received adjuvant radiotherapy for control of tumor residue, which was resected to Simpson grade II level. FRT is an accepted modality of treatment in SOMs as suggested by different authors.[
Limitations
We could not evaluate the impact of increasing expertise of the surgeon in surgically handling the cases during specific periods of time regarding the inequality between the numbers of cases undergoing surgery each year and the differences in the exact follow-up intervals.
CONCLUSION
Messages in this study can be:
A smaller (mini-) LO approach without pterional or orbito-basal craniotomy can be promising and enough for removal of hyperostosing SW meningiomas with orbital involvement in most of the cases (P = 0.54) This approach may be nominated as ‘less invasive’ due to smaller skin incision with most of it hidden within the eyebrow. The tumor can be exposed as a convexity meningioma without fronto-parieto-temporal craniotomy. Hereby, only the involved/reactive bone is removed. Bone should be excised as maximally as possible both to decompress the orbit appropriately and to minimize the chance of tumor recurrence (P = 0.37) A selected group of SWMs with tumor extension in the anterior part of the temporal fossa and in the lateral or superomedial compartment of the orbital cavity and ACP can be successfully approached through LO approach, thus avoiding craniotomy. In these cases, this minimally invasive procedure allows removal of the tumor mass and the infiltrated bone, with good clinical and cosmetic result (P = 0.97). Extension of the tumor mass medial to the axis of the optic nerve into the paranasal sinuses is considered the cut-off point for further dissection regarding the benign nature of the pathology and acceptable tumor control interval The thickened bone of the SW is not necessarily the origin of the tumor or infiltrated by the tumor (63%) but it can be a reactive sclerosing bone (27%) This surgical technique is certainly not advised in cases with a large, carpet like dural tumor growth extending over the convexity or passing midline in the skull base region It is suggested that the RIePM should be excluded from the standard grading system of SOMs (ePM) in future classifications of mid skull base meningiomas.
References
1. Abbott KH, Glass B. Pterional meningioma en plaque: Report of a case of thirty-six years’ duration. J Neurosurg. 1955. 12: 50-2
2. Al-Mefty O. Supraorbital-pterional approach to skull base lesions. Neurosurgery. 1987. 21: 474-7
3. Arai H, Sato K, Katsuta T, Rhoton AL. Lateral approach to intraorbital lesions: Anatomic and surgical considerations. Neurosurgery. 1996. 39: 1157-63
4. Baldeschi L, MacAndie K, Hintschich C, Wakelkamp IM, Prummel MF, Wiersinga WM. The removal of the deep lateral wall in orbital decompression: Its contribution to exophthalmos reduction and influence on consecutive diplopia. Am J Ophthalmol. 2005. 140: 642-7
5. Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007. 107: 905-12
6. Cannon PS, Rutherford SA, Richardson PL, King A, Leatherbarrow B. The surgical management and outcomes for spheno-orbital meningiomas. A 7-year review of multi-disciplinary practice. Orbit. 2009. 28: 371-6
7. Carrizo A, Basso A. Current surgical treatment for sphenoorbital meningiomas. Surg Neurol. 1998. 50: 574-8
8. Castellano F, Guidetti B, Olivecrona H. Pterional meningiomas en plaque. J Neurosurg. 1952. 9: 188-96
9. Civit T, Coinat-Coulbois S, Freppel S. Orbital metastasis. Neurochirurgie. 2010. 56: 148-51
10. Cophignon J, Lucena J, Clay C, Marchac D. Limits to radical treatment of spheno-orbital meningiomas. Acta Neurochir Suppl (Wien). 1979. 28: 375-80
11. Cristante L. Surgical treatment of meningiomas of the orbit and optic canal. A retrospective study with particular attention to the visual outcome. Acta Neurochir (Wien). 1994. 126: 27-32
12. Cushing H. The cranial hyperostosis produced by meningeal endotheliomas. Arch Neurol Psychiatry. 1922. 8: 139-54
13. Cushing H, Eisenhardt L.editors. The Meningiomas: Their classification, regional behavior, life history, and surgical end results. Springfield: Charles C. Thomas; 1938. p.
14. De Jesu×s O, Toledo MM. Surgical management of meningioma en plaque of the sphenoid ridge. Surg Neurol. 2001. 55: 265-9
15. Dolenc VV, Rogers L.editors. Evolution from the classical pterional to the contemporary approach to the central skull base. In Cavernous Sinus. Developments and Future Perspectives. New York: Springer; 2009. p. 61-74
16. Gaillard S, Lejeune JP, Pellerin P, Pertuzon B, Dhellemmes P, Christiansen JL. Long-term results of the surgical treatment of spheno-orbital osteomeningioma. Neurochirurgie. 1995. 4: 391-7
17. Goldberg RA, Kim AJ, Kerivan KM. The lacrimal keyhole, orbital door jamb and basin of the inferior orbital fissure. Three areas of deep bone in the lateral orbit. Arch Ophthalmol. 1998. 116: 1618-24
18. Goldsmith BJ, Wara WM, Wilson CB, Larson DA. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994. 80: 195-201
19. Heufelder MJ, Sterker I, Trantakis C, Schneider JP, Meixensberger J, Hemprich A. Reconstructive and ophthalmologic outcomes following resection of spheno-orbital meningiomas. Ophthal Plast Reconstr Surg. 2009. 5: 223-6
20. Honeybul S, Neil-Dwyer G, Lang DA, Evans BT, Ellison DW. Sphenoid wing meningioma en plaque: A clinical review. Acta Neurochir (Wien). 2001. 143: 749-58
21. Honig S, Trantakis C, Frerich B, Sterker I, Schober R, Meixensberger J. Spheno-orbital meningiomas: Outcome after microsurgical treatment: A clinical review of 30 cases. Neurol Res. 2010. 32: 314-25
22. Kim KS, Rogers LF, Goldblatt D. CT features of hyperostosing meningioma en plaque. AJR Am J Roentgenol. 1987. 149: 1017-23
23. Kim JW, Yates BS, Goldberg RA. Total lateral orbitotomy. Orbit. 2009. 28: 320-7
24. Langevin CJ, Hanasono MM, Riina HA, Stieg PE, Spinelli HM. Lateral transzygomatic approach to sphenoid wing meningiomas. Neurosurgery. 2010. 67: 377-84
25. Leake D, Gunnlaugsson C, Urban J, Marentette L. Reconstruction after resection of sphenoid wing meningiomas. Arch Facial Plast Surg. 2005. 7: 99-103
26. Li Y, Shi JT, An YZ, Zhang TM, Fu JD, Zhang JL. Sphenoid wing meningioma en plaque: Report of 37 cases. Chin Med J (Engl). 2009. 122: 2423-7
27. Mariniello G, Maiuri F, de Divitis E, Bonavolonta G, Tranfa F, Iuliano A. Lateral orbitotomy for removal of sphenoid wing meningiomas invading the orbit. Neurosurgery. 2010. 66: S287-92
28. Mariniello G, Maiuri F, Strianese D, Donzelli R, Iuliano A, Tranfa F. Spheno-orbital meningiomas: Surgical approaches and outcome according to the intraorbital tumor extent. Zentralbl Neurochir. 2008. 69: 175-81
29. Maroon JC, Kennerdell JS, Vidovich DV, Abla A, Sternau L. Recurrent spheno-orbital meningioma. J Neurosurg. 1994. 80: 202-8
30. McDermont MW, Durity FA, Rootman J, Woodhurst WB. Surgical technique. Combined frontotemporo-orbitozygomatic approach for tumors of the sphenoid wing and orbit. Neurosurgery. 1990. 26: 107-16
31. Miralbell R, Cella L, Weber D, Lomax A. Optimizing radiotherapy of orbital and paraorbital tumors: Intensity-modulated X-ray beams vs. intensity-modulated proton beams. Int J Radiat Oncol Biol Phys. 2000. 47: 1111-9
32. Mirone G, Chibbaro S, Schiabello L, Tola S, George B. En plaque sphemoid wing meningiomas: Recurrence factors and surgical strategy in a series of 71 patients. Neurosurgery. 2009. 65: S100-9
33. Nicolato A, Ferraresi P, Foroni R, Pasqualin A, Piovan E, Severi F. Gamma knife radiosurgery in skull base meningiomas. Preliminary experience with 50 cases. Stereotact Func Neurosurg. 1996. 66: 112-20
34. Nozaki K, Kikuta K, Takagi Y, Mineharu Y, Takahashi JA, Hashimoto N. Effect of early optic canal unroofing on the outcome of visual functions in surgery for meningiomas of the tuberculum sellae and planum sphenoidale. Neurosurgery. 2008. 62: 839-46
35. Oya S, Sade B, Lee JH. Sphenoorbital meningioma: Surgical technique and outcome. Clinical article. J Neurosurg. 2011. 114: 1241-9
36. Peele KA, Kennerdell JS, Maroon JC, Kalnicki S, Kazim M, Gardner T. The role of postoperative irradiation in the management of sphenoid wing meningiomas. A preliminary report. Ophthalmology. 1996. 103: 1761-7
37. Pompili A, Derome PJ, Visot A, Guiot G. Hyperostosing meningiomas of the sphenoid ridge-clinical features, surgical therapy, and long-term observations: Review of 49 cases. Surg Neurol. 1982. 17: 411-6
38. Ringel F, Cedzich C, Schramm J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery. 2007. 60: S214-21
39. Roser F, Nakamura M, Jacobs C, Vorkapic P, Samii M. Sphenoid wing meningiomas with osseous involvement. Surg Neurol. 2005. 64: 37-43
40. Saeed P, van Furth WR, Tanck M, Freling N, van der Sprenkel JW, Stalpers LJ. Surgical treatment of sphenoorbital meningiomas. Br J Ophthalmol. 2011. 95: 996-1000
41. Sandalcioglu IE, Gasser T, Mohr C, Stolke D, Wiedemayer H. Spheno-orbital meningiomas: Interdisciplinary surgical approach, resectability and long-term results. J Craniomaxillofac Surg. 2005. 33: 260-6
42. Scarone P, Leclerq D, He×ran F, Robert G. Long-term results with exophthalmos in a surgical series of 30 sphenoorbital meningiomas. Clinical article. J Neurosurg. 2009. 111: 1069-77
43. Schick U, Bleyen J, Bani A, Hassler W. Management of meningiomas en plaque of the sphenoid wing. J Neurosurg. 2006. 104: 208-14
44. Shrivastava RK, Sen C, Costantino PD, Della Rocca R. Sphenoorbital meningiomas: Surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005. 103: 491-7
45. Simas NM, Farias JP. Sphenoid wing en plaque meningiomas: Surgical results and recurrence rates. Surg Neurol Int. 2013. 4: 86-
46. Sincoff EH, Delashaw JB, Lee JH.editors. Orbitosphenoid meningiomas. Meningiomas. London: Springer-Verlag; 2008. p. 379-88