- Department of Neurosurgery, National Institute of Neurology and Neurosurgery, Manuel Velasco Suárez, Mexico City, Mexico.
- Department of Neuropathology, National Institute of Neurology and Neurosurgery, Manuel Velasco Suárez, Mexico City, Mexico.
Correspondence Address:
Edgar Nathal, Department of Vascular Neurosurgery, National Institute of Neurology and Neurosurgery, Manuel Velasco Suárez, Mexico City, Mexico.
DOI:10.25259/SNI_302_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rogelio Revuelta-Gutiérrez1, Alejandro Serrano-Rubio1, Rodrigo López-Rodríguez1, Héctor A. Rodríguez-Rubio1, Alfredo Bonilla-Suastegui1, Citlaltepetl Salinas Lara2, Edgar Nathal1. Lhermitte-Duclos disease: A rare case of cerebellar tumor with successful surgical treatment. 26-May-2023;14:185
How to cite this URL: Rogelio Revuelta-Gutiérrez1, Alejandro Serrano-Rubio1, Rodrigo López-Rodríguez1, Héctor A. Rodríguez-Rubio1, Alfredo Bonilla-Suastegui1, Citlaltepetl Salinas Lara2, Edgar Nathal1. Lhermitte-Duclos disease: A rare case of cerebellar tumor with successful surgical treatment. 26-May-2023;14:185. Available from: https://surgicalneurologyint.com/surgicalint-articles/12334/
Abstract
Background: Lhermitte-Duclos disease (LDD) or dysplastic gangliocytoma of the posterior fossa is a slow-growing and extremely rare mass lesion that involves the Purkinje neurons and the granular layer of the cerebellum. It is characterized by specific neuroradiological features and secondary hydrocephalus. However, documentation of surgical experience is scarce.
Case Description: A 54-year-old man with LDD manifesting as progressive headache is presented with vertigo and cerebellar ataxia. Magnetic resonance imaging demonstrated a right cerebellar mass lesion with the characteristic “tiger-striped appearance.” We decided to perform partial resection with reduction of tumor volume improving symptomatology as a result of the mass effect in the posterior fossa.
Conclusion: Surgical resection is a good alternative for the management of LDD, especially when neurological compromise exists due to mass effect.
Keywords: Cerebellum neoplasia, Dysplastic gangliocytoma, Hamartoma, Lhermitte-Duclos disease, Posterior fossa
INTRODUCTION
Lhermitte-Duclos disease (LDD), also known as dysplastic cerebellar gangliocytoma, is a rare and benign tumor of the cerebellum.[
CASE DESCRIPTION
A 54-year-old man with a history of systemic arterial hypertension was admitted to the hospital because of a sudden onset of the severe persistent occipital headache of 2 months of evolution accompanied by vertigo and cerebellar ataxia. The neurological examination revealed the presence of hypotonia of the right hemibody, limitation to infraduction and adduction of the right eye, and multidirectional nystagmus in both eyes. Signs and symptoms compatible with increased intracranial pressure were also found, such as headache, nausea, and vomiting; no clinical data were found such as macroglossia, polydactyly, gigantism, or hydromyelia. Initial brain MRI showed a right cerebellar hemisphere sharply defined mass of 7.6 cm × 7.6 cm × 8.9 cm, with a prominent striated foliar pattern, causing obstructive hydrocephalus. The T1 appeared a hypointense, and T2 was a hyperintense-weighted image with parallel linear striations with a characteristic “tiger-striped appearance” visible on T2-weighted imaging [
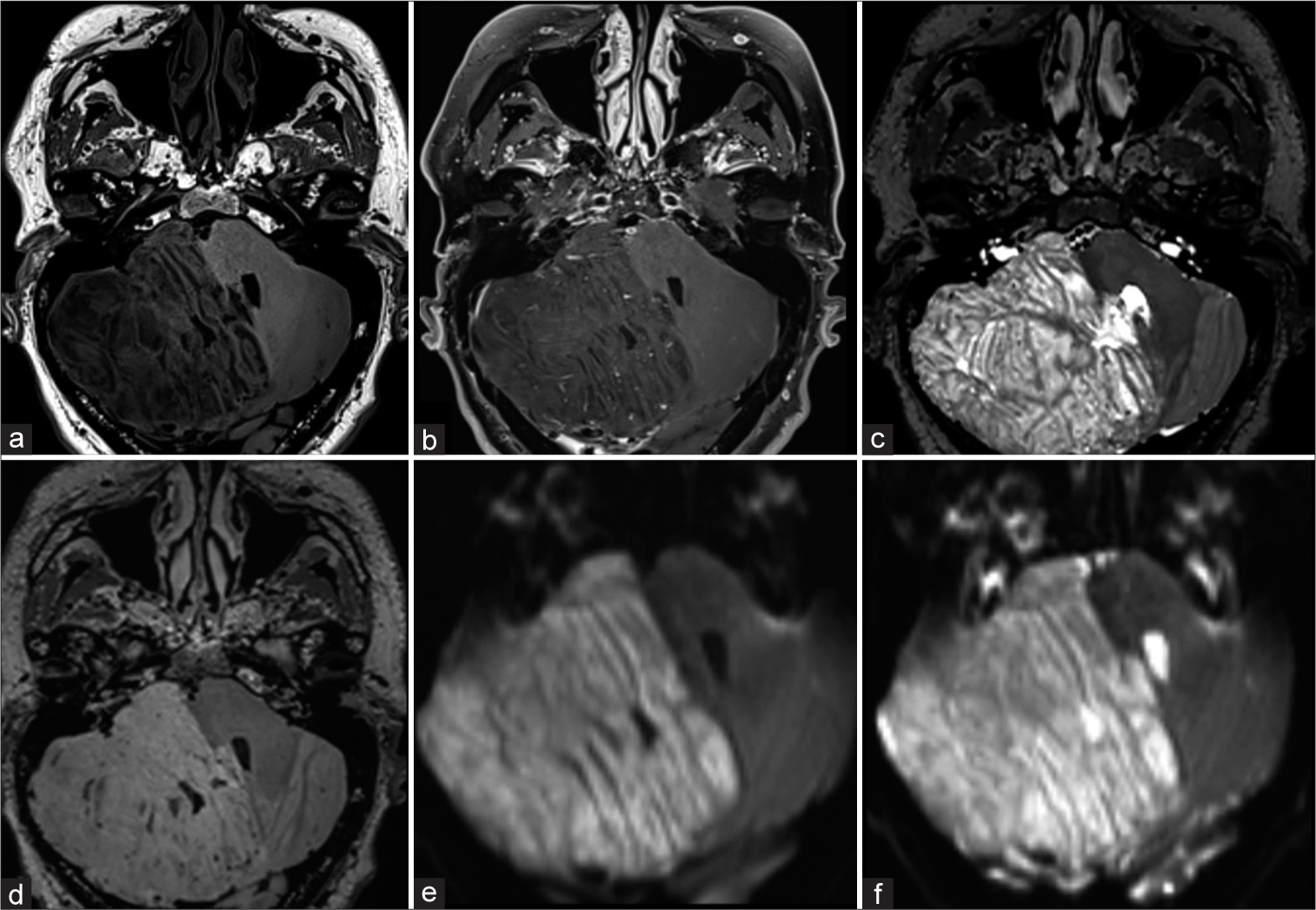
Figure 1:
Preoperative axial magnetic resonance imaging. It shows a predominantly right hemispheric lesion, a characteristic laminar appearance is observed due to abnormal thickening of the cerebellar folia (“tiger-stripe appearance”). In T1-weighted (a) the lesion shows hypointensity, in the contrasted T1-weighted (b) no contrast-enhancement, no edema in T2-weighted (c) and fluid attenuated inversion recovery-weighted (d), no diffusion restriction, and no calcifications or hemorrhage in the susceptibility (e and f).
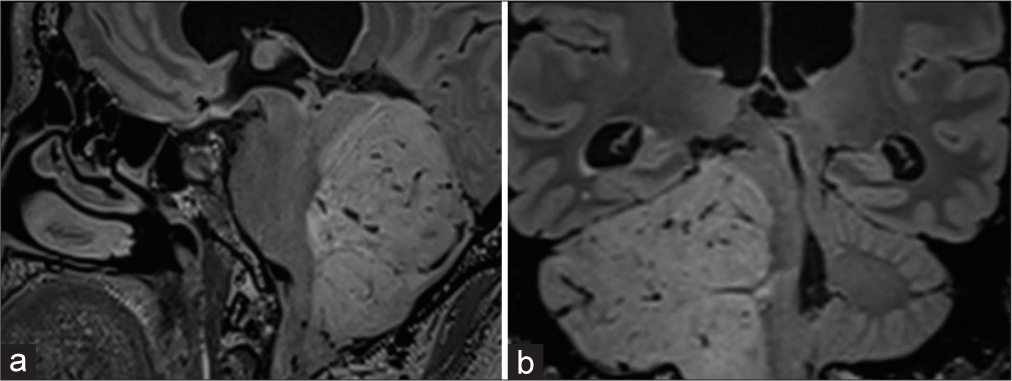
Figure 2:
Magnetic resonance imaging sagittal and coronal projections. (a and b) fluid attenuated inversion recovery-weighted; infiltration is observed in the inferior, middle, and superior cerebellar peduncles, displacing the fourth ventricle and narrowing the anterior and lateral cisterns of the posterior fossa.
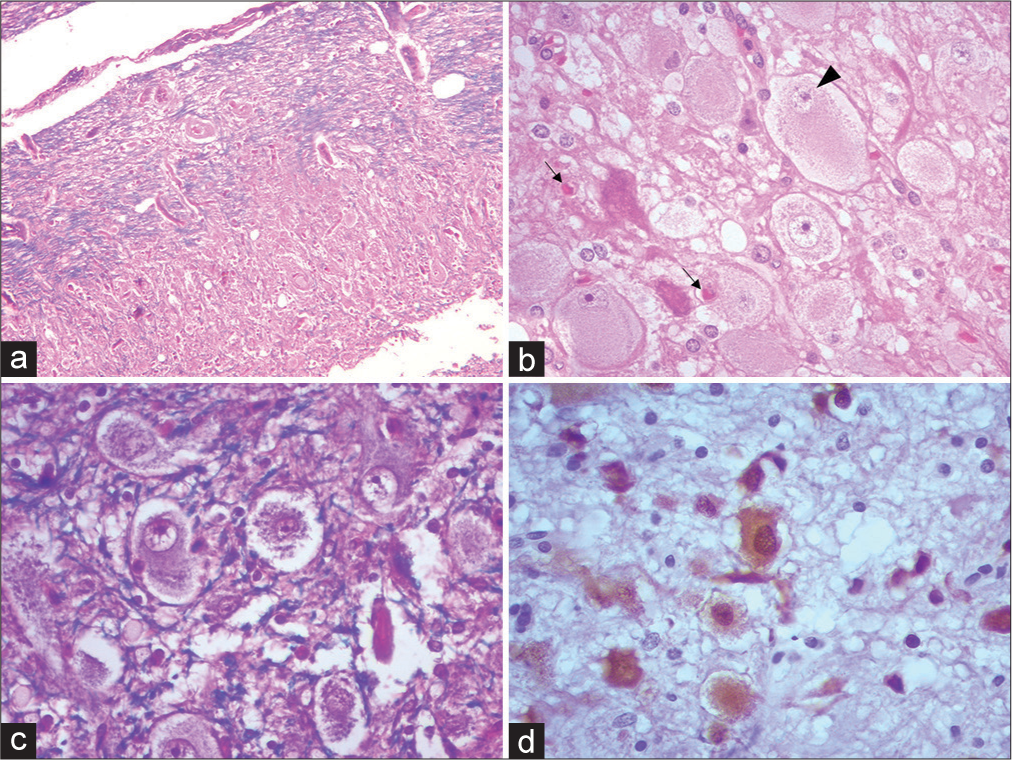
Figure 3:
Photomicrographs of Lhermitte-Duclos disease showing the spectrum of changes. (a) Cerebellar cortex replaced by the presence of dysplastic cells characteristic of the disease, encompassing virtually all three histologic strata. H and E ×100 (b) Cluster of dysplastic cells with a large eosinophilic cytoplasm of globoid appearance, with a large nucleus, slightly recessed to the periphery. (Black arrowhead) Amphophilic nucleolus. (black arrows) Open neuropil, with edema and vacuolation. Alternates with apoptotic appearing neurons. H and E ×400 (c) KluverBarrera/H and E staining showing the direction in different directions of axons and loss of layering of neuronal somas. Note that the fibers are tortuous, serpiginous, and disorganized (blue). ×400 (d) Neuronal bodies of different sizes and morphology (brown). In addition, little gliosis or satellitosis was evident. IHC NeuN ×400.
DISCUSSION
The incidence is extremely rare and not reported. The disease should be suspected in a young adult in the third or fourth decade of life who presents with clinical signs and symptoms of a progressive posterior fossa mass.[
CONCLUSION
This case represents a diagnostic challenge due to its low frequency. This case documents the surgical and functional success obtained by the patient, resolving his symptoms and his disease. Surgical resection is a good alternative for the management of LDD, especially when neurological compromise exists due to mass effect.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Almubarak AO, Haq AU, Alzahrani I, Shail EA. LhermitteDuclos disease with cervical arteriovenous fistula. J Neurol Surg A Cent Eur Neurosurg. 2019. 80: 134-7
2. Andres RH, Guzman R, Weis J, Brekenfeld C, Fandino J, Seiler RW. Lhermitte-Duclos disease with atypical vascularization--case report and review of the literature. Clin Neuropathol. 2009. 28: 83-90
3. Ashraf M, Kamboh UA, Raza MA, Choudhary N, Mehboob M, Hussain SS. Lhermitte-Duclos disease: A rare cerebellar hamartoma presenting following traumatic brain injury and a review of the literature. J Ayub Med Coll Abbottabad. 2022. 34: S733-8
4. Biswas SN, Chakraborty PP, Patra S. Lhermitte-Duclos disease. BMJ Case Rep. 2016. 2016: bcr2015214235
5. Chourafa Z, Kissani N, Louhab N. Lhermitte-Duclos disease at an initial stage: Case report. Rev Neurol (Paris). 2021. 177: 1042-4
6. Giannini C, Scheithauer BW, Lloyd RV, editors. Lhermitte-Duclos Disease. Pathology and Genetics of Tumours of the Nervous System. Lyon: IARC Press; 2000. p. 210-1
7. Kulkantrakorn K, Awwad EE, Levy B, Selhorst JB, Cole HO, Leake D. MRI in Lhermitte-Duclos disease. Neurology. 1997. 48: 725-31
8. Kumar R, Vaid VK, Kalra SK. Lhermitte-Duclos disease. Childs Nerv Syst. 2007. 23: 729-32
9. Kumar S, Sahana D, Jain A, Rathore L, Tawari M, Mittal J. Lhermitte Duclos disease: What to expect during surgery?. Egypt J Neurosurg. 2021. 36: 40
10. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization Classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
11. Marsh DJ, Coulon V, Lunetta KL, Rocca-Serra P, Dahia PL, Zheng Z. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet. 1998. 7: 507-15
12. Meltzer CC, Smirniotopoulos JG, Jones RV. The striated cerebellum: An MR imaging sign in Lhermitte-Duclos disease (dysplastic gangliocytoma). Radiology. 1995. 194: 699-703
13. Niemann S, Mühlen AZ, Dörner E. Lhermitte-Duclos disease: A report of two cases and review of the literature. Acta Neurochir (Wien). 2017. 159: 1875-81
14. Nowak DA, Trost HA. Lhermitte-Duclos disease (dysplastic cerebellar gangliocytoma): A malformation, hamartoma or neoplasm?. Acta Neurol Scand. 2002. 105: 137-45
15. Pandey S, Sarma N. Lhermitte-Duclos disease: A rare cause of cerebellar ataxia. Asian J Neurosurg. 2017. 12: 705-6
16. Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: Systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013. 105: 1607-16
17. Pregúntegui-Loayza I, Apaza-Tintaya A, Ramírez-Espinoza A, Mayo-Simón N, Toledo-Aguirre M. Lhermitte-Duclos disease in pediatric population: Report of 2 cases. Pediatr Neurosurg. 2021. 56: 279-85
18. Turnbull MM, Humeniuk V, Stein B, Suthers GK. Arteriovenous malformations in Cowden syndrome. J Med Genet. 2005. 42: e50
19. Van Lieshout A, Gielens MP, Noordveld RB. Lhermitte-Duclos disease. JBR BTR. 2014. 97: 178-9
20. Wan X, Chu H, Chen W, Wang F. Dysplastic cerebellar gangliocytoma: A description of two cases. Quant Imaging Med Surg. 2021. 11: 4695-9