- Department of Neurosurgery, Hospital Universitário Evangelico de Curitiba, Curitiba, Parana, Brazil.
Correspondence Address:
Irlon Oliveira, Department of Neurosurgery, Hospital Universitário Evangelico de Curitiba, Curitiba, Parana, Brazil.
DOI:10.25259/SNI_52_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Luis A. B. Borba, Gustavo Passos, Irlon Oliveira. Liquid biopsy and tumor DNA/RNA detection in the cerebrospinal fluid of patients diagnosed with central nervous system glioma – A review article. 26-May-2023;14:183
How to cite this URL: Luis A. B. Borba, Gustavo Passos, Irlon Oliveira. Liquid biopsy and tumor DNA/RNA detection in the cerebrospinal fluid of patients diagnosed with central nervous system glioma – A review article. 26-May-2023;14:183. Available from: https://surgicalneurologyint.com/surgicalint-articles/12336/
Abstract
Background: Gliomas are the most common primary malignant neoplasms of the central nervous system and their characteristic genetic heterogeneity implies in a prominent complexity in their management. The definition of the genetic/molecular profile of gliomas is currently essential for the classification of the disease, prognosis, choice of treatment, and it is still dependent on surgical biopsies, which in many cases become unfeasible. Liquid biopsy with detection and analysis of biomarkers such as deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) from the tumor and circulating in the bloodstream or cerebrospinal fluid (CSF) has emerged as a minimally invasive alternative to aid in diagnosis, follow-up, and response to treatment of gliomas.
Methods: Through a systematic search in the PubMed MEDLINE, Cochrane Library, and Embase databases, we reviewed the evidence on the use of liquid biopsy to detect tumor DNA/RNA in the CSF of patients diagnosed with central nervous system gliomas.
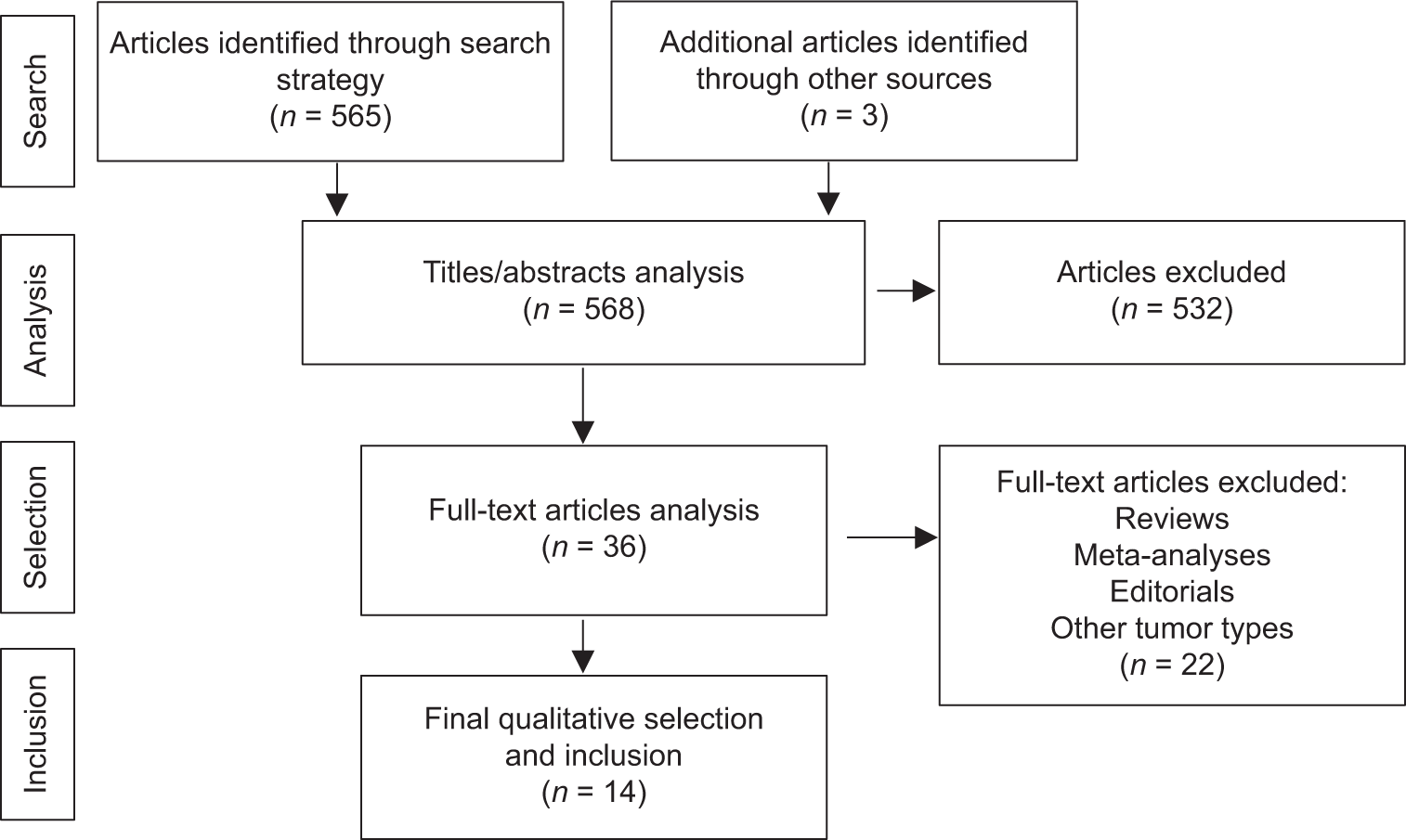
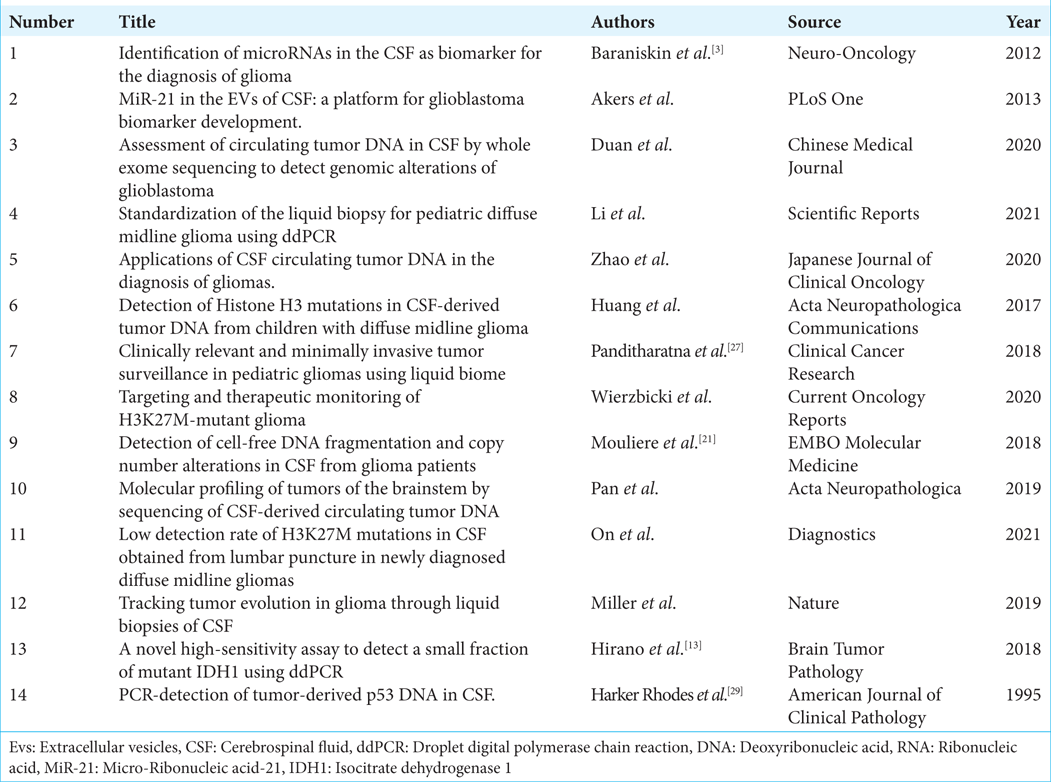
Results: After a systematic review applying all inclusion and exclusion criteria, as well as a double review by independent authors, 14 studies specifically addressing the detection of tumor DNA/RNA in the CSF of patients diagnosed with central nervous system glioma were selected in the final analysis.
Conclusion: Sensitivity and specificity of liquid biopsy in CSF are still very variable depending on factors such as the diagnostic method, collection timing, biomarker (DNA and RNA), tumor type, extension and volume of the tumor, collection method, and contiguity from neoplasm to CSF. Despite the technical limitations that still exist and prevent the routine and validated use of liquid biopsy in CSF, the growing number of studies around the world is increasingly improving this technic, resulting in promising prospects for its use in diagnosis, evolutionary follow-up, and response to the treatment of complex diseases such as central nervous system gliomas.
Keywords: Cerebrospinal fluid, Circulating tumor DNA, Circulating Tumor RNA, Gliomas, Liquid biopsy
INTRODUCTION
Central nervous system gliomas represent a complex and heterogeneous disease with multiple variants that affect from the pediatric age group to seniors. They represent about 25.1% of all primary tumors in the central nervous system and 80.8% of malignant brain tumors[
In this review, through a systematic search in PubMed databases MEDLINE, Cochrane Library, and Embase, we reviewed evidence from studies that address the application of liquid biopsy in the detection of tumor circulating DNA or RNA in the CSF of patients diagnosed with gliomas of the central nervous system.
Objectives
The aim of the study was to assess the evidence on the effectiveness of liquid biopsy and detection of tumor DNA/ RNA in the CSF of patients with central nervous system gliomas.
MATERIALS AND METHODS
Literature search strategy
The search was performed on PubMed MEDLINE, Cochrane Library, and Embase. The descriptors used in the formulation of the search strategy were defined based on the DECS/ MESH structured health vocabulary and systematized to increase the sensitivity of the initial research. The descriptors used were: “Liquid biopsy” [MeSH Terms] AND “CSF” [MeSH Terms] AND “circulating tumor DNA/CSF” [MeSH Terms] OR “circulating microrna/CSF” [MeSH Terms] OR “cell-free DNA” [MeSH Terms] OR “tumor derived DNA” [MeSH Terms] AND “glioma” [MeSH Terms] OR “glioma/ CSF” [MeSH Terms].
We do not set limits for the start date of publications, while to the final date, we established the limitation until October, 2021. After the initial research, two reviewers chose the relevant publications for the review based on the titles and abstracts found. Then, the full texts of the selected publications were reviewed to determine those compatible with the inclusion and exclusion criteria. In addition, we examined the references of the selected studies to verify the existence of other studies compatible with the search strategy but which were not included in the initial research.
Inclusion criteria
Studies were selected according to the following inclusion criteria: studies with liquid biopsy in CSF, patients diagnosed with central nervous system gliomas, and studies in English language.
Exclusion criteria
Studies were selected according to the following exclusion criteria: studies without availability of abstract, studies with other types of tumors, systematic reviews, meta-analyses, editorials, studies with exclusive blood plasma analysis, and animal studies.
RESULTS
DISCUSSION
Central nervous system gliomas represent complex neoplasms and mostly with a poor prognosis. The knowledge of its genetic heterogeneity has been decisive for the understanding of oncogenesis and consequently for the development of new treatment options and survival improvement. Liquid biopsy of CSF has emerged as a promising tool in the management of gliomas, providing information that helps in the diagnosis, definition of the genetic profile of the disease, and response to treatment.
In the pediatric subgroup, the importance and utility of liquid biopsy are highlighted due to the higher prevalence of midline gliomas, especially of the brain stem as these neoplastic subtypes usually imply difficulties for surgical biopsy, with limited treatment options and dismal prognosis.[
Although there are still many limitations and challenges, in recent years, the amount of research dedicated to the study of tumor biomarkers present in the CSF and improvement of diagnostic techniques has grown dramatically. It is known that tumor DNA/RNA is difficult to detect in CSF due to its fragmentation into small chains, short half-life,[
In our review, we found that the diagnostic sensitivity of liquid biopsy in CSF is still very variable depending on factors such as the diagnostic method, collection timing, biomarker (DNA, RNA), tumor type, extension and volume of the tumor, collection method, and contiguity from neoplasm to CSF.[
Although our review focused on analyzing the use of tumor DNA/RNA, we found that other biomarkers have shown promise in the CSF liquid biopsy of patients diagnosed with gliomas. Due to the relevance of the various biomarkers researched, below we highlight some characteristics of those most used today:
Circulating tumor DNA
Small fragments of 150–200 base pairs of tumor-derived DNA, circulating in the bloodstream or CSF and not coupled to cells.[
Circulating tumor RNA
This group includes micro-RNAs, long noncoding RNAs, and small non-coding RNAs.[
EVs
Both tumor cells and normal cells secrete EVs that carry diverse contents including proteins, lipids, DNA, and RNA that seem to perform functions of intercellular communication and regulation.[
Circulating tumor cells
Cells derived from the primary tumor and that enter the bloodstream or CSF are called circulating tumor cells.[
Proteins
Genetic alterations in neoplasms such as gliomas alter the expression of cellular proteins and consequently define specific profiles that can be used as tumor biomarkers for diagnostic, therapeutic, and prognostic purposes.[
CONCLUSION
Through this review, we investigated the effectiveness of liquid biopsy and detection of tumor DNA/RNA in the CSF of patients diagnosed with gliomas of the central nervous system. The diagnostic sensitivity and specificity of liquid biopsy in CSF are still very variable depending on factors such as the diagnostic method, collection timing, biomarker (DNA and RNA), tumor type, extension and volume of the tumor, collection method, and contiguity from neoplasm to CSF. Despite the technical limitations that still exist and prevent the validated and routine use of liquid biopsy in CSF, the growing number of studies around the world increasingly improves it, resulting in promising perspectives for its use in diagnosis, evolutionary follow-up, and response to the treatment of complex diseases such as central nervous system gliomas.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Akers JC, Ramakrishnan V, Kim R, Skog J, Nakano I, Pingle S. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): A platform for glioblastoma biomarker development. PLoS One. 2013. 8: e78115
2. Azad TD, Jin MC, Bernhardt LJ, Bettegowda C. Liquid biopsy for pediatric diffuse midline glioma: A review of circulating tumor DNA and cerebrospinal fluid tumor DNA. Neurosurg Focus. 2020. 48: E9
3. Baraniskin A, Kuhnhenn J, Schlegel U, Maghnouj A, Zöllner H, Schmiegel W. Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro Oncol. 2012. 14: 29-33
4. Bark JM, Kulasinghe A, Chua B, Day BW, Punyadeera C. Circulating biomarkers in patients with glioblastoma. Br J Cancer. 2020. 122: 295-305
5. Berger A, Santic R, Almer D, Hauser-Kronberger C, Huemer M, Humpel C. Galanin and galanin receptors in human gliomas. Acta Neuropathol. 2003. 105: 555-60
6. Birkó Z, Nagy B, Klekner Á Virga J. Novel molecular markers in glioblastoma-benefits of liquid biopsy. Int J Mol Sci. 2020. 21: 7522
7. De Mattos-Arruda L, Mayor R, Ng CK, Weigelt B, MartínezRicarte F, Torrejon D. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015. 6: 8839
8. Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008. 14: 985-90
9. Dietz MS, Beach CZ, Barajas R, Parappilly MS, Sengupta SK, Baird LC. Measure twice: Promise of liquid biopsy in pediatric high-grade gliomas. Adv Radiat Oncol. 2020. 5: 152-62
10. Duan H, Hu JL, Chen ZH, Li JH, He ZQ, Wang ZN. Assessment of circulating tumor DNA in cerebrospinal fluid by whole exome sequencing to detect genomic alterations of glioblastoma. Chin Med J (Engl). 2020. 133: 1415-21
11. Francis G, Stein S. Circulating cell-free tumour DNA in the management of cancer. Int J Mol Sci. 2015. 16: 14122-42
12. Garcia CM, Toms SA. The role of circulating MicroRNA in glioblastoma liquid biopsy. World Neurosurg. 2020. 138: 425-35
13. Hirano M, Ohka F, Maeda S, Chalise L, Yamamichi A, Aoki K. A novel high-sensitivity assay to detect a small fraction of mutant IDH1 using droplet digital PCR. Brain Tumor Pathol. 2018. 35: 97-105
14. Huang SW, Ali ND, Zhong L, Shi J. MicroRNAs as biomarkers for human glioblastoma: Progress and potential. Acta Pharmacol Sin. 2018. 39: 1405-13
15. Huang TY, Piunti A, Lulla RR, Qi J, Horbinski CM, Tomita T. Detection of Histone H3 mutations in cerebrospinal fluid-derived tumor DNA from children with diffuse midline glioma. Acta Neuropathol Commun. 2017. 5: 28
16. Kang Y, Lin X, Kang D. Diagnostic value of circulating tumor DNA in molecular characterization of glioma: A meta-analysis. Medicine (Baltimore). 2020. 99: e21196
17. Khalil AA. Biomarker discovery: A proteomic approach for brain cancer profiling. Cancer Sci. 2007. 98: 201-13
18. Klekner Á, Szivos L, Virga J, Árkosy P, Bognár L, Birkó Z. Significance of liquid biopsy in glioblastoma-A review. J Biotechnol. 2019. 298: 82-7
19. Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014. 3: 26913
20. Miller AM, Shah RH, Pentsova EI, Pourmaleki M, Briggs S, Distefano N. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019. 565: 654-8
21. Mouliere F, Mair R, Chandrananda D, Marass F, Smith CG, Su J. Detection of cell-free DNA fragmentation and copy number alterations in cerebrospinal fluid from glioma patients. EMBO Mol Med. 2018. 10: e9323
22. Müller C, Holtschmidt J, Auer M, Heitzer E, Lamszus K, Schulte A. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med. 2014. 6: 247ra101
23. On J, Natsumeda M, Watanabe J, Saito S, Kanemaru Y, Abe H. Low detection rate of H3K27M mutations in cerebrospinal fluid obtained from lumbar puncture in newly diagnosed diffuse midline gliomas. Diagnostics (Basel). 2021. 11: 681
24. Osti D, Del Bene M, Rappa G, Santos M, Matafora V, Richichi C. Clinical significance of extracellular vesicles in plasma from glioblastoma patients. Clin Cancer Res. 2019. 25: 266-76
25. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020. 22: v1-96
26. Pan C, Diplas BH, Chen X, Wu Y, Xiao X, Jiang L. Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol. 2019. 137: 297-306
27. Panditharatna E, Kilburn LB, Aboian MS, Kambhampati M, Gordish-Dressman H, Magge SN. Clinically relevant and minimally invasive tumor surveillance of pediatric diffuse midline gliomas using patient-derived liquid biopsy. Clin Cancer Res. 2018. 24: 5850-9
28. Qu S, Guan J, Liu Y. Identification of microRNAs as novel biomarkers for glioma detection: A meta-analysis based on 11 articles. J Neurol Sci. 2015. 348: 181-7
29. Rhodes CH, Honsinger C, Sorenson GD. PCR-detection of tumor-derived p53 DNA in cerebrospinal fluid. Am J Clin Pathol. 1995. 103: 404-8
30. Rolhion C, Penault-Llorca F, Kémény JL, Lemaire JJ, Jullien C, Labit-Bouvier C. Interleukin-6 overexpression as a marker of malignancy in human gliomas. J Neurosurg. 2001. 94: 97-101
31. Shen F, Zhang Y, Yao Y, Hua W, Zhang HS, Wu JS. Proteomic analysis of cerebrospinal fluid: Toward the identification of biomarkers for gliomas. Neurosurg Rev. 2014. 37: 367-80
32. Simonelli M, Dipasquale A, Orzan F, Lorenzi E, Persico P, Navarria P. Cerebrospinal fluid tumor DNA for liquid biopsy in glioma patients’ management: Close to the clinic?. Crit Rev Oncol Hematol. 2020. 146: 102879
33. Theeler BJ, Yung WK, Fuller GN, De Groot JF. Moving toward molecular classification of diffuse gliomas in adults. Neurology. 2012. 79: 1917-26
34. Westphal M, Lamszus K. Circulating biomarkers for gliomas. Nat Rev Neurol. 2015. 11: 556-66
35. Wierzbicki K, Ravi K, Franson A, Bruzek A, Cantor E, Harris M. Targeting and therapeutic monitoring of H3K27M-mutant glioma. Curr Oncol Rep. 2020. 22: 19
36. Zhang W, Zhang J, Hoadley K, Kushwaha D, Ramakrishnan V, Li S. miR-181d: A predictive glioblastoma biomarker that downregulates MGMT expression. Neuro Oncol. 2012. 14: 712-9
37. Zhao Z, Zhang C, Li M, Shen Y, Feng S, Liu J. Applications of cerebrospinal fluid circulating tumor DNA in the diagnosis of gliomas. Jpn J Clin Oncol. 2020. 50: 325-32