- Department of Neurosurgery, Kindai University Faculty of Medicine, Osaka, Japan
- Department of Microbiology, Kindai University Faculty of Medicine, Osaka, Japan
Correspondence Address:
Hisashi Kubota
Department of Microbiology, Kindai University Faculty of Medicine, Osaka, Japan
DOI:10.4103/sni.sni_195_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hisashi Kubota, Yasuhiro Sanada, Saori Murakami, Masaharu Miyauchi, Michihiro Iwakura, Kazuhiro Nagatsuka, Kentaro Furukawa, Amami Kato, Mitsugu Fujita. Long-term follow-up for ossification of autologous bone plug and skin sinking after periosteum-preserved burr hole surgery. 06-Sep-2017;8:204
How to cite this URL: Hisashi Kubota, Yasuhiro Sanada, Saori Murakami, Masaharu Miyauchi, Michihiro Iwakura, Kazuhiro Nagatsuka, Kentaro Furukawa, Amami Kato, Mitsugu Fujita. Long-term follow-up for ossification of autologous bone plug and skin sinking after periosteum-preserved burr hole surgery. 06-Sep-2017;8:204. Available from: http://surgicalneurologyint.com/surgicalint-articles/long%e2%80%91term-follow%e2%80%91up-for-ossification-of-autologous-bone-plug-and-skin-sinking-after-periosteum%e2%80%91preserved-burr-hole-surgery/
Abstract
Background:The demand of a burr hole surgery for chronic subdural hematoma (CSDH) is increasing in the global aging society. Burr hole-derived autologous bone dusts are not associated with extra costs compared with other commonly used synthetic materials. In addition, postoperative calvarium ossification requires periosteum-mediated blood supply, which is lacking after using avascular synthetic materials. Based on these findings, we hypothesized that the combination of the bone plugs and the preserved periosteum during burr hole surgeries for CSDH would induce efficient calvarium ossification.
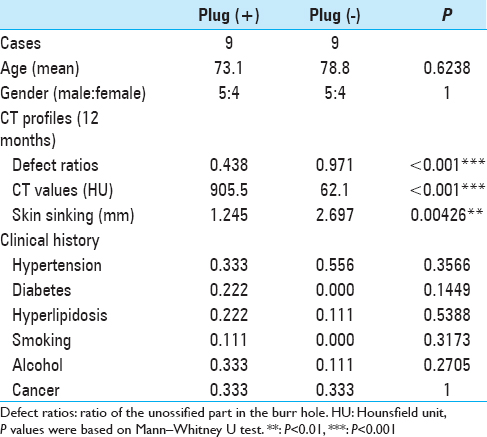
Methods:We evaluated the long-term effects of bone plugs on the degree of ossification and cosmetic appearance of the skin covering the burr hole sites. We included 8 patients (9 burr holes) who received the autologous bone dust derived from burr holes. As the control group, 9 burr holes that did not receive any burr hole plugs were retrospectively selected. These burr holes were evaluated by computed tomography (CT) scan for the calvarium defect ratios, CT value-based ossification, and the degree of skin sinking.
Results:Ossification was observed in all the bone plugs by the bone density CT scans; they maintained their volume at 12 months after the surgeries. The calvarium defect ratios (volume ratios of the unossified parts in the burr holes) gradually increased during the first 6 months and reached 0.44 at 12 months. The mean CT values also increased from 527 HU to 750 HU for the first 6 months and reached 905 HU at 12 months. The degrees of skin sinking at the burr hole sites with the bone plugs were 1.24 mm whereas those without the bone plugs were 2.69 mm (P = 0.004).
Conclusion:Application of burr hole-derived autologous bone dust is associated with better ossification and objective cosmetic result following burr hole surgery after CSDH.
Keywords: Bone plug, burr hole reconstruction, intramembrane ossification, periosteum, skin sinking
INTRODUCTION
Chronic subdural hematoma (CSDH) is becoming one of the most frequent neurosurgical operations in the world, mainly due to ageing population but also due to the increased use of antithrombotic medications.[
MATERIALS AND METHODS
Patient profiles
This study has been approved by the ethical committee of our institute, and the informed consent was obtained from all the patients included in the study. The burr hole surgery for CSDH performed at the department of neurosurgery in Kindai University Hospital (Osaka-Sayama, Japan) from February 2014 to August 2015. Consecutive eight patients received nine burr hole surgeries, performed by the first author, with the same number of the bone plugs (one patient presented bilateral chronic subdural hematoma) [
Burr hole surgery
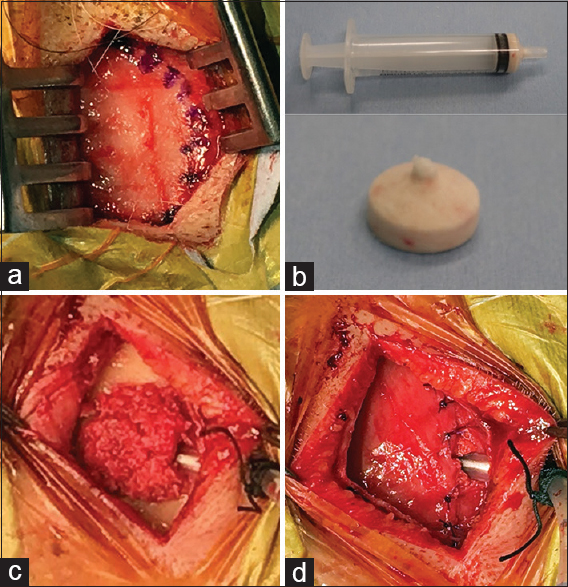
For these patients, a single burr hole surgery was performed as follows [
Figure 1
Process of burr hole reconstruction using an autologous bone plug. (a) Periosteum is carefully preserved and undermined under the subgaleal space. Periosteal incision is presented (dot line). (b) The bone plug is created with strong pressure using a 5 ml syringe. (c) The drainage tube is inserted, and then the burr hole is filled with the bone plug. (d) The bone plug is completely covered with the periosteum sutured in place
CT scan-based evaluation
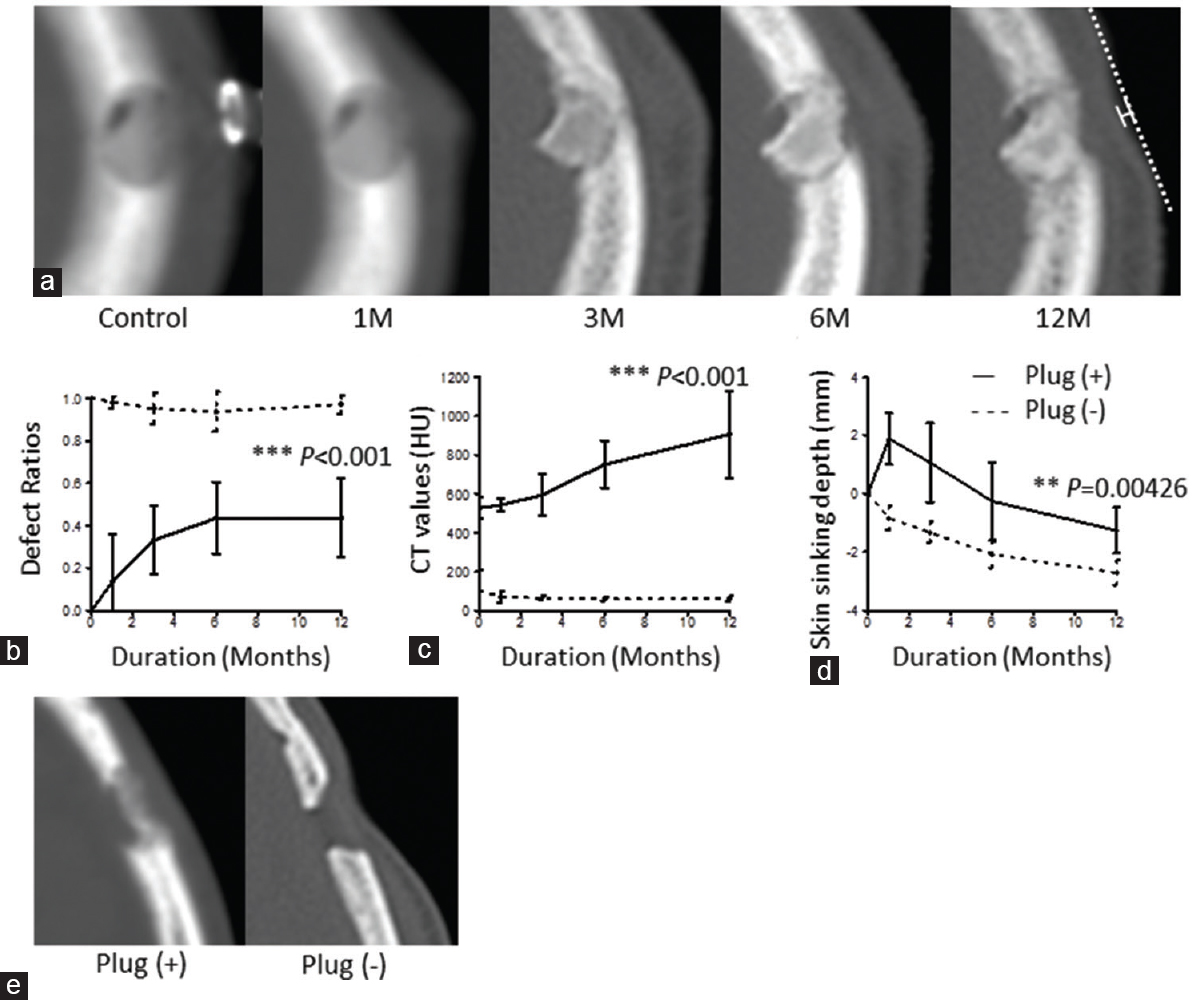
All the patients underwent CT scans temporally after the surgery [
Figure 2
Consecutive changes of the volume and CT values of bone plugs, and the degree of skin sinking at 1 month and 12 months after burr hole surgery. (a) A representative case is presented. Method for measurement of skin sinking on CT scan is shown. A natural curve is drawn through the edges of a skin deposit, and the perpendicular length from the chord is measured. (b) Each volume of the calvarium defect ratios by burr holes is plotted during the postoperative time course (solid line). The defect ratios without bone plugs is plotted in the same way (dashed line). (c) Each CT value is plotted in two groups with (solid line) or without the bone plugs (dashed line). (d) The degree of skin sinking is plotted and compared among the patients with the bone plugs (solid line) and those without the bone plugs (dashed line). Error bars represent standard deviations. (e) Skin sinking with or without the bone plug at 12 months after a surgery. (b-d) P values are based on Mann–Whitney U test. **: P <0.01, ***: P <0.001
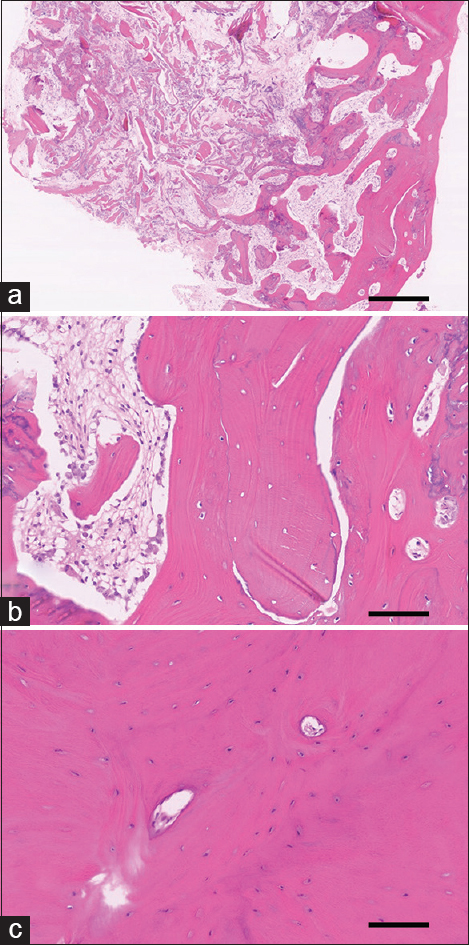
Histopathological evaluation
Hematoxylin-eosin (HE) staining was conducted according to a previous study[
Figure 3
Hematoxylin-eosin staining of the bone plug revealed its re-ossification at 6 months after the implantation. (a) Bone fragments are observed along with fibrous tissue. Mature bone components are partially observed. Original magnification: 40×. Scale bar: 500 μm. (b) Magnified view. Bone spicules and osteocytes are observed. Lining of osteoblasts is also observed. Original magnification: 200×. Scale bar: 100 μm. (c) Intact cortical bone. Osseous lamella and osteocytes but no fibrous components are observed. Original magnification: 200×. Scale bar: 100 μm
Statistical analyses
Statistical analyses were performed on R Environment (R Development Core Team, Vienna, Austria) with EZR plugin.[
RESULTS
Both groups, with or without the bone plugs, comprised 9 patients (5 males and 4 females) [
The volume of the calvarium defects by burr holes ranged 237–1150 mm3. The calvarium defect ratios of those with the bone plugs exhibited approximately 0.33 in 3 months after the surgery and 0.44 in 6 months and 12 months [
The CT values on the following day of the surgery ranged between 427 HU and 585 HU [
Skin sinking at the burr hole sites with the bone plugs reverted to the level of normal skin levels at 6 months after the surgery and slightly progressed at 12 months, whereas skin sinking of those without the bone plugs began immediately after the surgery and steadily progressed until 12 months [
HE staining of the bone plug at 6 months after the surgery revealed the lining of osteoblasts, osteocytes, and bone spicules. Fibrous tissues and fibroblasts were also observed, although no inflammatory responses were observed [Figure
DISCUSSION
In this study, we have demonstrated the effectiveness of bone plugs created from burr hole-derived bone dusts to reconstruct calvarium. The use of bone dusts/fibrin glue mixture has been previously reported to induce ossification at the burr hole site as well as the bone gaps created by craniotomy.[
We also observed the duration of ossification mediated by the bone plugs. The first step of ossification is resorption of bone graft; the bone plugs without fibrin glue exhibited a significant decrease in volume for the first 3 months. In contrast, regarding the fibrin-fixed bone dust strategy, the fibrin glue was absorbed for the first 2 months, and the bone dusts were absorbed for the first 3 months, which is almost consistent with the observation of the bone plugs. The difference between these strategies is the second step – osteoblastic activation and bone deposition. Physical fixation of the bone dusts increases the density and interaction of osteogenic components even without the use of fibrin glue, which promotes its resorption and triggers subsequent osteogenesis. Consistently, we observed an increase in CT values during the follow-up periods and the formation of firmed bone matrix at 6 months after the surgery. Our results (approximately 60% volume retention of bone plugs) are consistent with a previous study based on a rodent model.[
There are two suggested mechanisms of ossification – intramembranous ossification and endochondral ossification.[
Current practice dictates that, to prevent skin sinking followed by burr hole surgery, foreign materials such as a hydroxyapatite are widely used to fill and cover burr holes.[
The present study has several limitations. The biggest limitation is that this technique was applied only for the patients the first author operated, which resulted in a paucity of experimental cases. Thus, we need to accumulate the cases to perform valid statistical analyses. In addition, although we showed 1-year outcome of this technique in this study, the long-term outcome also needs to be elucidated in the future.
It is of note that increasing demands for burr hole surgeries for CSDH are inevitable in a global aging society. An autologous bone plug might offer the resolution of not only cosmetic issues relevant to the burr hole surgeries but also prevention for excess usage of foreign materials. In conclusion, the use of autologous bone plug created by bone dusts might be a simple, inexpensive, and cosmetically excellent method to prevent a skin sinking after burr hole surgeries.
CONCLUSION
Application of burr hole-derived autologous bone dust is associated with better ossification and objective cosmetic result following burr hole surgery after CSDH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bostrom S, Kourtopoulos H, Nilsson I. Reconstruction of craniotomy burr-holes with autologous bone blugs made by a new hole-saw. Acta Neurochir (Wien). 1990. 105: 132-4
2. Bostrom S, Zsigmond P, Karlsson A, Nilsson I. A new bone dust packer for use in neurosurgery. Acta Neurochir (Wien). 1999. 141: 183-5
3. DeLuca L, Raszewski R, Tresser N, Guyuron B. The fate of preserved autogenous bone graft. Plast Reconstr Surg. 1997. 99: 1324-8
4. Guha D, Coyne S, Macdonald RL. Timing of the resumption of antithrombotic agents following surgical evacuation of chronic subdural hematomas: A retrospective cohort study. J Neurosurg. 2016. 124: 750-9
5. Gupta A, Lobocki C, Malhotra G, Jackson IT. Comparison of osteogenic potential of calvarial bone dust, bone fragments, and periosteum. J Craniofac Surg. 2009. 20: 1995-9
6. Im TS, Lee YS, Suh SJ, Lee JH, Ryu KY, Kang DG. The Efficacy of Titanium Burr Hole Cover for Reconstruction of Skull Defect after Burr Hole Trephination of Chronic Subdural Hematoma. Korean J Neurotrauma. 2014. 10: 76-81
7. Kashimura H, Ogasawara K, Kubo Y, Yoshida K, Sugawara A, Ogawa A. A newly designed hydroxyapatite ceramic burr-hole button. Vasc Health Risk Manag. 2010. 6: 105-8
8. Karamese M, Toksoz MR, Selimoglu MN, Akdag O, Toy H, Tosun Z. Comparison of bone dust with other types of bone grafts for cranioplasty. J Craniofac Surg. 2014. 25: 1155-8
9. Koyama J, Hongo K, Iwashita T, Kobayashi S. A newly designed key-hole button. J Neurosurg. 2000. 93: 506-8
10. Matsumoto K, Kohmura E, Kato A, Hayakawa T. Restoration of small bone defects at craniotomy using autologous bone dust and fibrin glue. Surg Neurol. 1998. 50: 344-6
11. Matsuo K, Koizumi K, Fujita M, Morikawa T, Jo M, Shibahara N. Efficient Use of a Crude Drug/Herb Library Reveals Ephedra Herb As a Specific Antagonist for TH2-Specific Chemokine Receptors CCR3, CCR4, and CCR8. Front Cell Dev Biol. 2016. 4: 54-
12. Spotnitz WD. Fibrin Sealant: The Only Approved Hemostat, Sealant, and Adhesive-a Laboratory and Clinical Perspective. ISRN Surg. 2014. p. 203943-
13. Tasaki T, Fujita M, Okuda T, Yoneshige A, Nakata S, Yamashita K. MET Expressed in Glioma Stem Cells Is a Potent Therapeutic Target for Glioblastoma Multiforme. Anticancer Res. 2016. 36: 3571-7
14. Tubbs RS, Bosmia AN, Cohen-Gadol AA. The human calvaria: A review of embryology, anatomy, pathology, and molecular development. Childs Nerv Syst. 2012. 28: 23-31
15. Wan Q, Schoemaker T, Jansen ID, Bian Z, de Vries TJ, Everts V. Osteoblasts of calvaria induce higher numbers of osteoclasts than osteoblasts from long bone. Bone. 2016. 86: 10-21
16. Worm PV, Ferreira NP, Faria MB, Ferreira MP, Kraemer JL, Collares MV. Comparative study between cortical bone graft versus bone dust for reconstruction of cranial burr holes. Surg Neurol Int. 2010. 1: 91-
17. Yamashima T. Reconstruction of surgical skull defects with hydroxylapatite ceramic buttons and granules. Acta Neurochir (Wien). 1988. 90: 157-62
18. Yoshioka H, Okuda T, Fujita M, Inoue T, Tasaki T, Izumoto S. Inhibition of ABCG2 enhances chemo-sensitivity of murine glioma stem cell-like cells to temozolomide and reduces spheroid-forming capability. Acta Med Kinki Univ. 2014. 39: 105-13