- Department of Neurosurgery, Akita University Graduate School of Medicine, Akita, Japan.
- Department of Neuro-Oncology/Neurosurgery, Saitama Medical University International Medical Center, Hidaka, Japan.
- Department of Neurosurgery, Kitasato University School of Medicine, Sagamihara, Japan.
Correspondence Address:
Takahiro Ono, Department of Neurosurgery, Akita University Graduate School of Medicine, Akita, Japan.
DOI:10.25259/SNI_1141_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takahiro Ono1, Haruka Kuwashige1, Jun-Ichi Adachi2, Masataka Takahashi1, Masaya Oda1, Toshihiro Kumabe3, Hiroaki Shimizu1. Long-term survival of a patient with diffuse midline glioma in the pineal region: A case report and literature review. 14-Dec-2021;12:612
How to cite this URL: Takahiro Ono1, Haruka Kuwashige1, Jun-Ichi Adachi2, Masataka Takahashi1, Masaya Oda1, Toshihiro Kumabe3, Hiroaki Shimizu1. Long-term survival of a patient with diffuse midline glioma in the pineal region: A case report and literature review. 14-Dec-2021;12:612. Available from: https://surgicalneurologyint.com/surgicalint-articles/11287/
Abstract
Background: Diffuse midline glioma (DMG) is an invasive astrocytic tumor arisen from midline structures, such as the pons and thalamus. Five cases of DMG in the pineal region have been reported, but the clinical course was poor; there was no case of survival for more than 2 years.
Case Description: We report the case of a 12-year-old boy with DMG in the pineal region who is living a normal daily life for more than 6 years following multimodal treatment. He complained of a headache accompanied by vomiting that had gradually worsened 1 month previously, and initial magnetic resonance imaging revealed a pineal tumor. Germinoma was initially suspected; however, a combination of chemotherapy using carboplatin and etoposide was ineffective. The first surgery was performed through the left occipital transtentorial approach (OTA); the diagnosis was DMG. After 60 Gy radiotherapy concomitant with temozolomide (TMZ), the tumor enlarged. Second surgery was performed through bilateral OTAs, and 90% of the tumor was removed. In addition, stereotactic radiotherapy (30 Gy, six fractions) was administered, and the local equivalent dose in 2 Gy/fraction reached 97.5 Gy. Maintenance chemotherapy using TMZ and bevacizumab was continued for 2 years. After finishing chemotherapy, the enhancing lesion enlarged again, and bevacizumab monotherapy was effective. Now, at 6 years after diagnosis, the patient leads an ordinary life as a student.
Conclusion: Maximum resection and high-dose radiotherapy followed by bevacizumab may have been effective in the present case.
Keywords: Bevacizumab, Diffuse midline glioma, High-dose radiotherapy, Maximum resection, Pineal tumors
INTRODUCTION
Diffuse midline glioma (DMG) commonly develops in midline structures, such as the pons and thalamus, and is molecularly characterized by H3.3 or H3.1 K27M mutation.[
We herein report the case of a patient with DMG extended to the pineal region who has lived for more than 6 years following multimodal treatment.
CASE DESCRIPTION
A 12-year-old boy, who had no notable medical, family, or psychosocial history, presented with headache and vomiting. Magnetic resonance imaging (MRI) revealed hydrocephalus and a pineal region tumor with a major axis of 34 mm. Serum levels of alpha-fetoprotein and beta-human chorionic gonadotropin were within the normal range. Initially, the tumor was suspected of germinoma; hence, endoscopic biopsy and third ventriculostomy were performed. Although hydrocephalus improved, histological diagnosis could not be obtained.
Combination chemotherapy using carboplatin and etoposide was administered as a diagnostic treatment. However, the tumor had grown 1 month after (maximum diameter, 46 mm) [
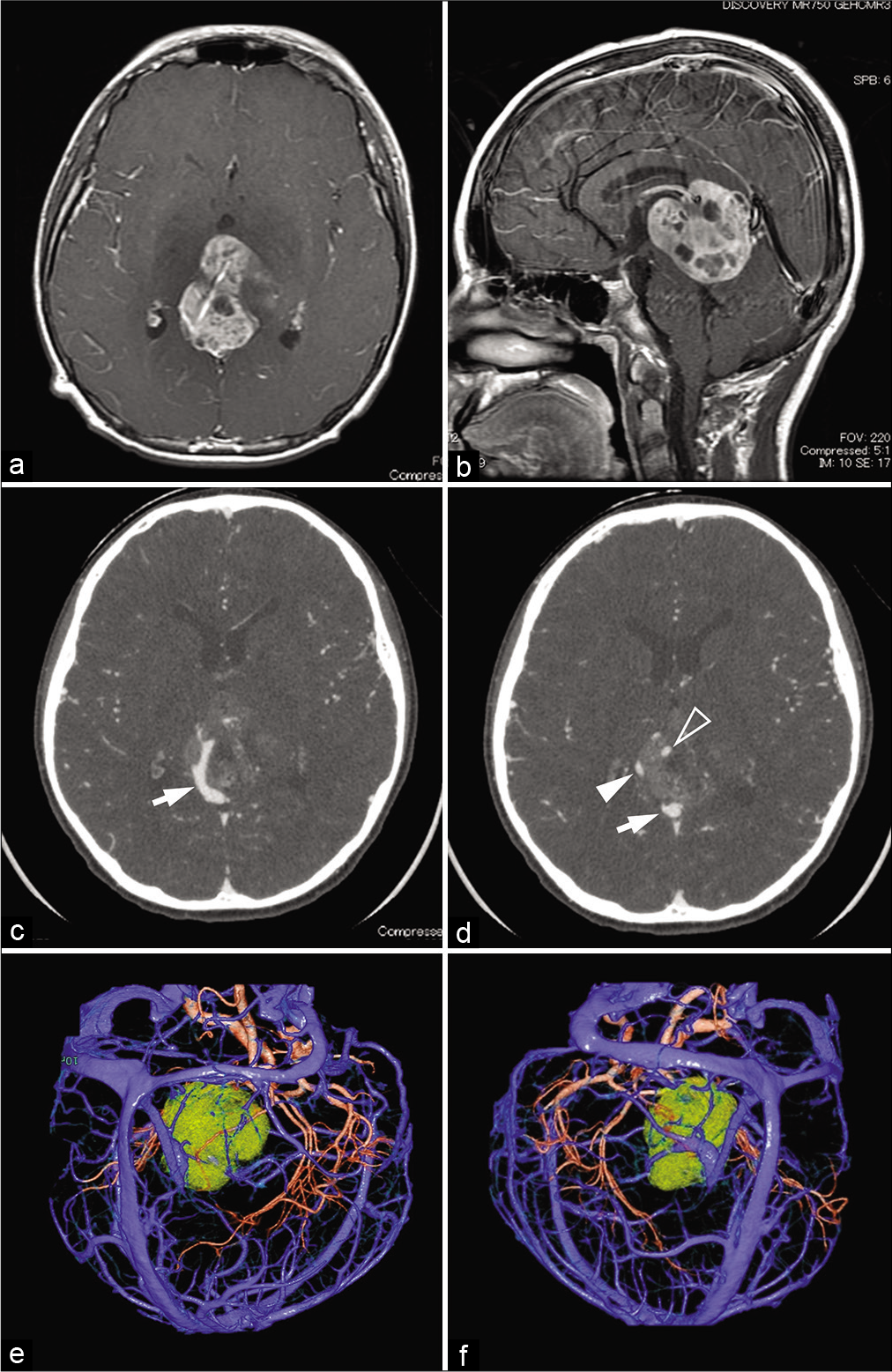
Figure 1:
(a and b) Axial and sagittal post contrast T1-weighted images. A pineal region tumor had grown after combination chemotherapy using carboplatin and etoposide, with a maximum diameter of 46 mm. (c and d) Axial source images of preoperative three-dimensional computed tomography angiography (3D-CTA). Arrows (c) and a solid, open arrowhead (d) show the vein of Galen and the right and left internal cerebral vein, respectively; the left internal cerebral vein is passing through the tumor. (e and f) Reconstructed 3D-CTA images for preoperative planning indicate the left (e) and right (f) occipital transtentorial approaches, respectively.
Preoperative three-dimensional computed tomography angiography revealed that the right internal cerebral vein (ICV) and the vein of Galen had shifted to the right side, and the left ICV passed through the tumor [
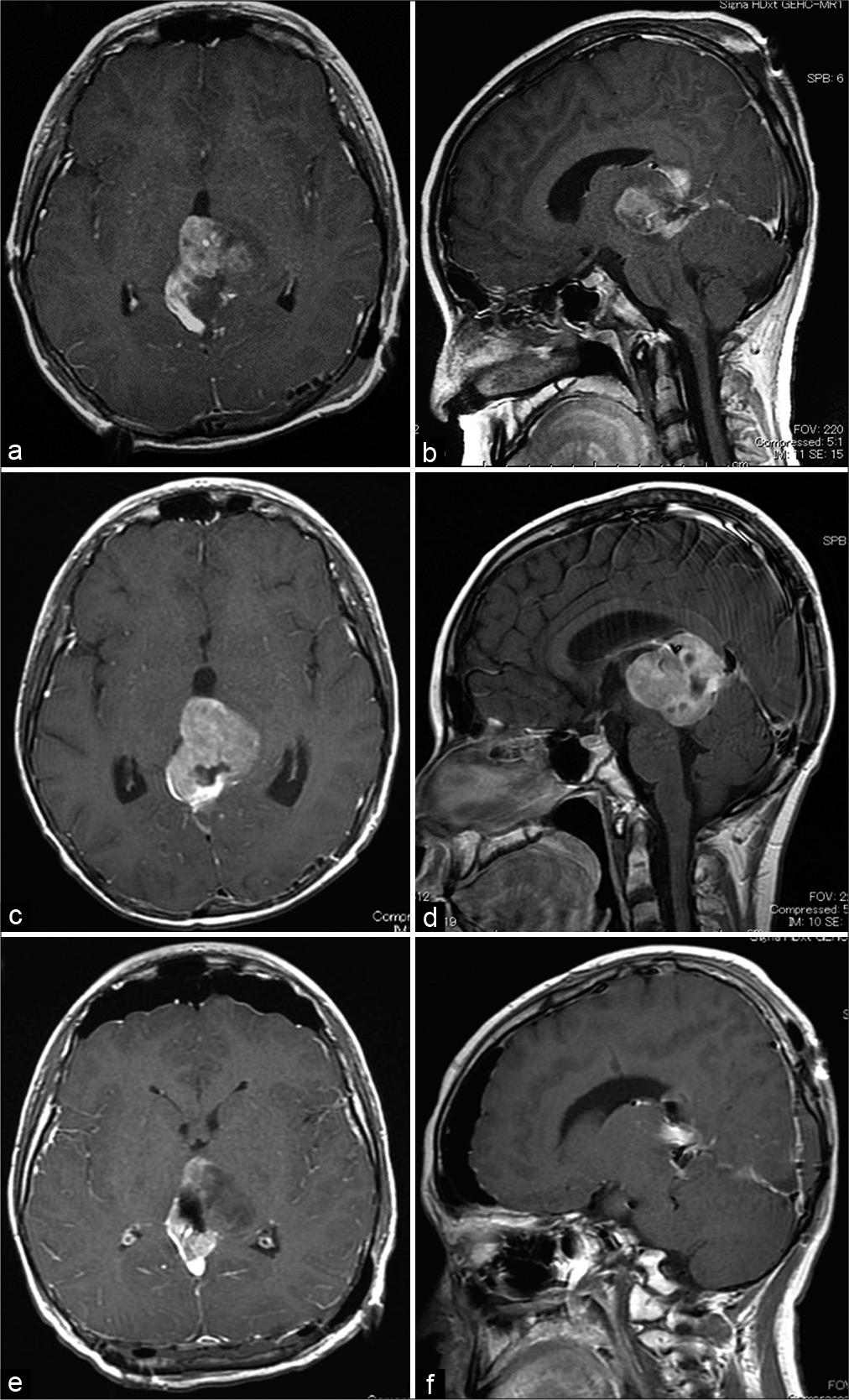
Figure 2:
Axial and sagittal post contrast T1-weighted images. (a and b) The tumor was partially removed in the initial surgery by the left occipital transtentorial approach. (c and d) After surgery, whole-brain radiotherapy (26 Gy) and local radiotherapy (34 Gy) were performed concomitantly with temozolomide administration; however, the residual tumor enlarged. (e and f) Subsequently, the second surgery was performed using bilateral occipital transtentorial approach, and approximately 90% of the tumor volume was removed.
Based on the 2007 WHO classification, the tumor was classified as a malignant pineal parenchymal tumor or malignant glioma at our hospital and was diagnosed as a malignant glioma through a central review. Subclassification was difficult because of chemotherapy-induced tissue degeneration.
During the central review, postoperative therapy was initiated with whole-brain irradiation (26 Gy, 2 Gy/fraction) and switched to local irradiation (34 Gy) concomitant with temozolomide (TMZ). However, post treatment MRI revealed tumor regrowth (maximum diameter, 47 mm) [
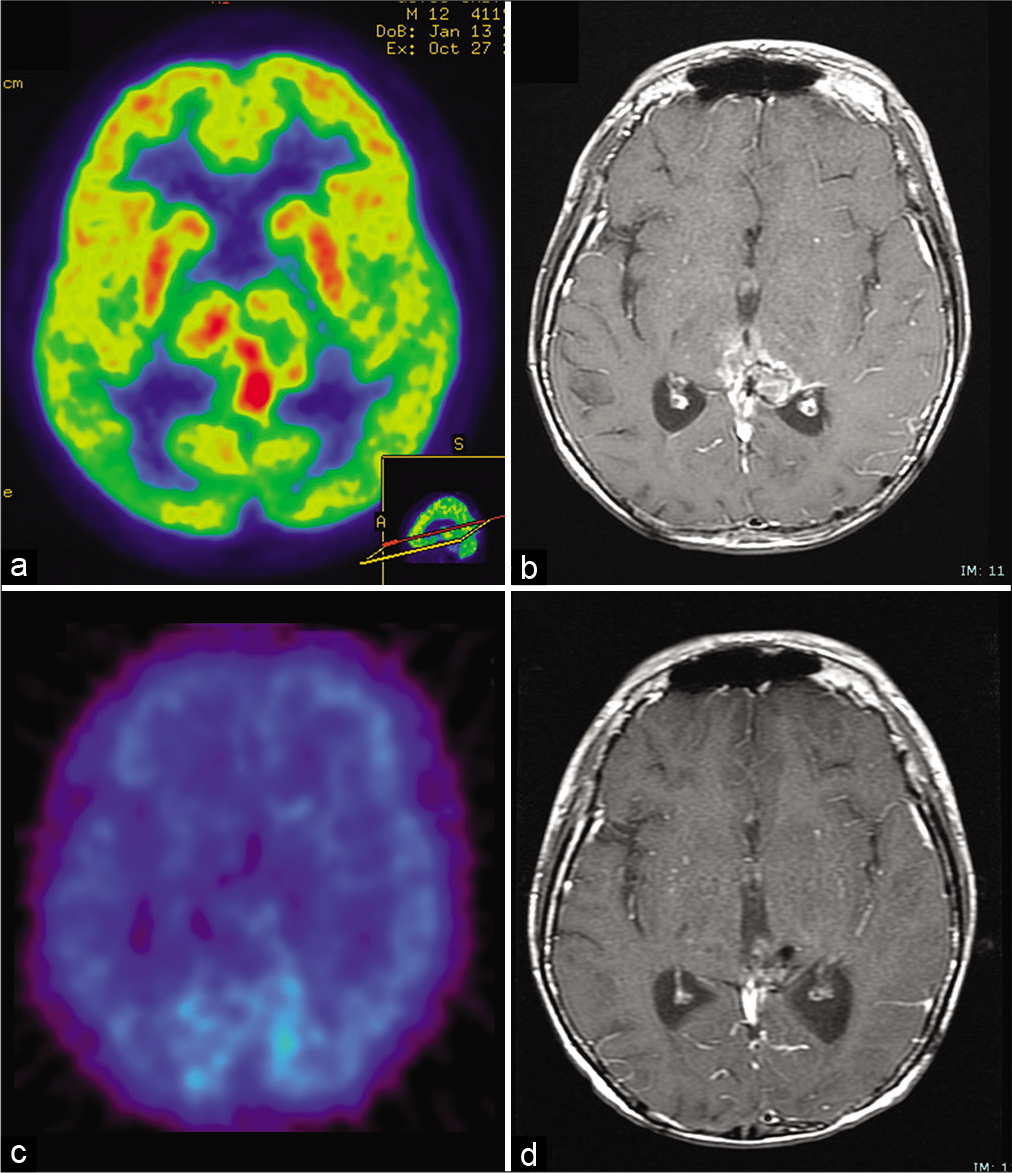
One month after discharge, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) revealed high glucose metabolism in the residual tumor [
Figure 3:
(a) 18F-fluorodeoxyglucose positron emission tomography 6 weeks after the second surgery showing high glucose metabolism in the residual tumor. (b) Post contrast T1-weighted images obtained 2 months after the end of maintenance chemotherapy (29 months after initial surgery) showing enlargement of the enhancing lesion. (c) However, 11C-methionine positron emission tomography demonstrated no abnormal metabolism. (d) Post contrast T1-weighted images reveals no recurrence under bevacizumab monotherapy 6 years after initial surgery.
MRI performed 2 months after the end of chemotherapy (29 months after the first surgery) again revealed an enlargement in the enhanced area [
Pathological review
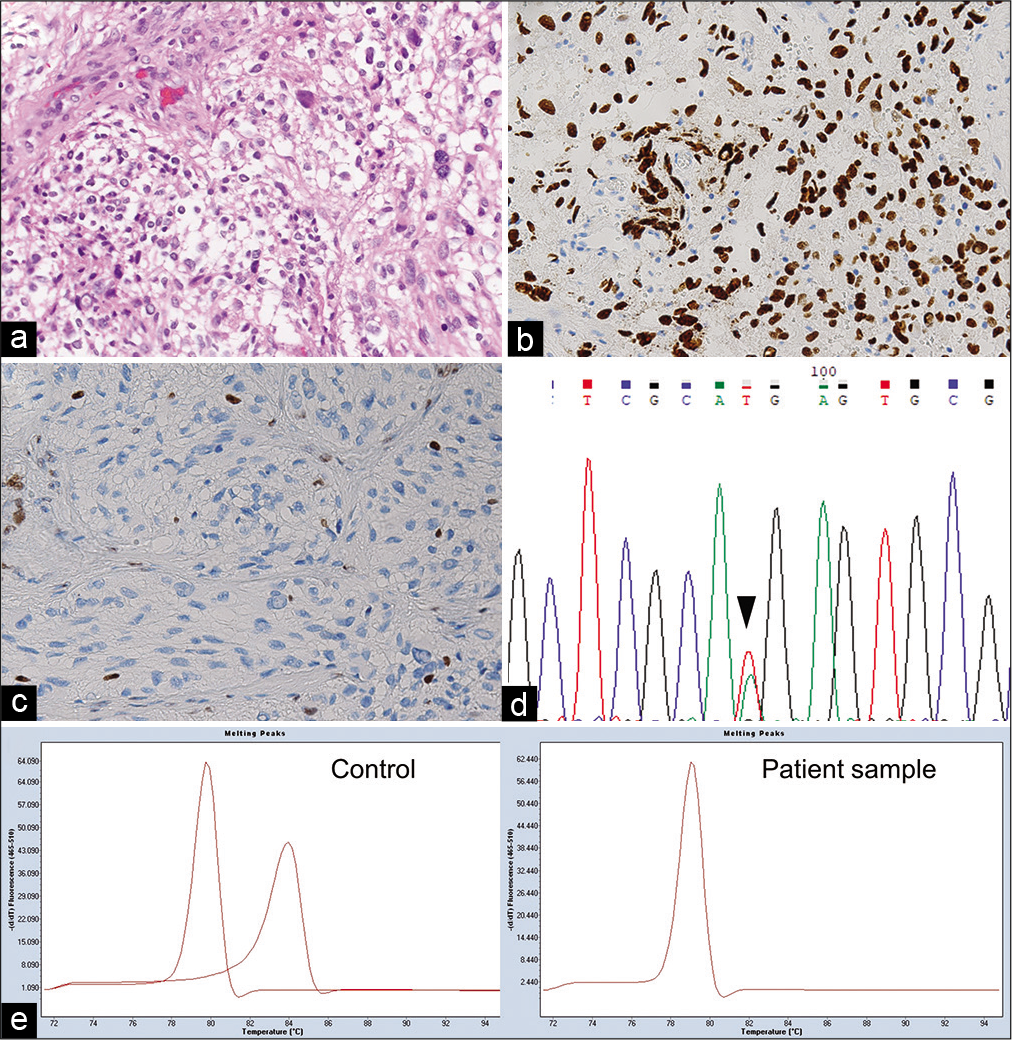
Written informed consent for molecular analyzes and publication was obtained. The sample from the first surgical removal was histologically investigated according to the 2021 WHO classification. Hematoxylin-eosin staining [
Figure 4:
Pathological and molecular findings of the present tumor. (a) Hematoxylin and eosin staining showing marked diffuse proliferation of the atypical astrocytic tumor cells. (b and c) Immunohistochemical staining showing that the tumor cells were positive for H3.3 K27M and negative for H3 K27me3. (d) Sequencing chromatogram, obtained using Sanger sequencing, showing K27M mutation in H3F3A (c. 83A>T) (arrowhead). (e) MGMT methylation-sensitive high-resolution melting (MSHRM) analysis showing two peaks of 0% (left) and 100% (right) methylated controls in the left panel. Two different peaks are obtained for the PCR product derived from the unmethylated and methylated templates. The right panel is from the sample of the present case demonstrating a peak of unmethylated DNA. Data were analyzed using the Tm Calling software module.
The mutations of IDH1/2, H3.3 encoded by H3F3A, and BRAF were screened by the high-resolution melting (HRM) analysis[
DISCUSSION
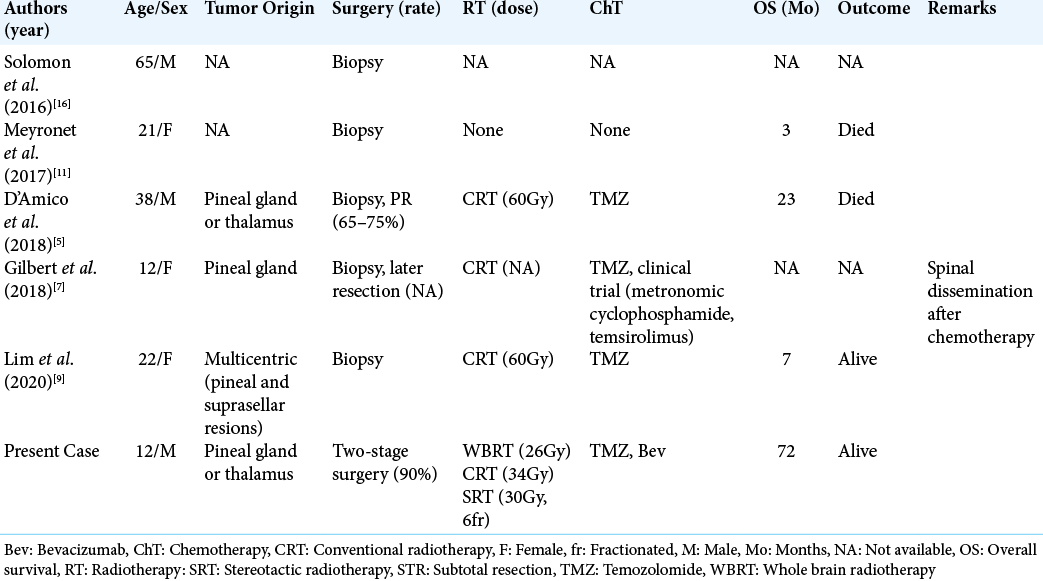
We report a case of DMG in the pineal region with a long-term survival of 6 years after multimodal treatment. To date, five cases of pineal DMG have been reported [
Most DMGs do not have methylation in the MGMT gene promoter, and TMZ has poor treatment efficacy.[
In the present case, 90% of the tumor was removed through two operations. According to the previous reports, a tumor removal of 78% or more contributes to better prognosis in patients with malignant gliomas.[
Another feature in this case was high-dose radiation therapy with a total dose of 97.5 Gy. In general, local irradiation with a total dose of 60 Gy is recommended for glioblastoma. Similar treatment has been applied for DMG in the pineal region previously; however, the clinical outcome has been unsatisfactory.[
The high-dose radiation therapy carries the risk of late complications, including leukoencephalopathy, cerebral atrophy, and radiation necrosis.[
CONCLUSION
We report a patient with DMG in the pineal region who had a long-term survival of more than 6 years. Maximum resection through bilateral OTA and high-dose radiotherapy followed by Bev might have contributed to the outcome of the present case.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to sincerely thank Dr. Yoichi Nakazato, professor emeritus at the Department of Pathology, Gunma University Graduate School of Medicine, for conducting the central pathological diagnosis.
References
1. Adachi JI, Mishima K, Suzuki T, Fujimaki T, Nishikawa R. Rapid IDH1 gene mutation analysis for intraoperative pathological diagnosis. Neuro Oncol. 2014. 16: iii47
2. Adachi J, Mishima K, Wakiya K, Suzuki T, Fukuoka K, Yanagisawa T. O6-methylguanine-DNA methyltransferase promoter methylation in 45 primary central nervous system lymphomas: Quantitative assessment of methylation and response to temozolomide treatment. J Neurooncol. 2012. 107: 147-53
3. Castel D, Philippe C, Calmon R, Le Dret L, Truffaux N, Boddaert N. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015. 130: 815-27
4. Cohen KJ, Heideman RL, Zhou T, Holmes EJ, Lavey RS, Bouffet E. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: A report from the children’s oncology group. Neuro Oncol. 2011. 13: 410-6
5. D’Amico RS, Zanazzi G, Wu P, Canoll P, Bruce JN. Pineal region glioblastomas display features of diffuse midline and non-midline gliomas. J Neurooncol. 2018. 140: 63-73
6. Furuse M, Nonoguchi N, Kuroiwa T, Miyamoto S, Arakawa Y, Shinoda J. A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis. Neurooncol Pract. 2016. 3: 272-80
7. Gilbert AR, Zaky W, Gokden M, Fuller CE, Ocal E, Leeds NE. Extending the neuroanatomic territory of diffuse midline glioma, K27M mutant: Pineal region origin. Pediatr Neurosurg. 2018. 53: 59-63
8. Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011. 79: 1487-95
9. Lim JX, Leong A, Tan AP, Tan CL, Nga VD. H3K27M-mutant diffuse midline glioma presenting as synchronous lesions involving pineal and suprasellar region: A case report and literature review. J Clin Neurosci. 2020. 81: 144-8
10. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
11. Meyronet D, Esteban-Mader M, Bonnet C, Joly MO, Uro-Coste E, Amiel-Benouaich A. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 2017. 19: 1127-34
12. Murphy ES, Xie H, Merchant TE, Yu JS, Chao ST, Suh JH. Review of cranial radiotherapy-induced vasculopathy. J Neurooncol. 2015. 122: 421-9
13. Qiu B, Wang Y, Ou S, Guo Z, Wang Y. The unilateral occipital transtentorial approach for pineal region meningiomas: A report of 15 cases. Int J Neurosci. 2014. 124: 741-7
14. Romanelli P, Conti A, Pontoriero A, Ricciardi GK, Tomasello F, de Renzis C. Role of stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of recurrent glioblastoma multiforme. Neurosurg Focus. 2009. 27: E8
15. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011. 115: 3-8
16. Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJ. Diffuse midline gliomas with histone H3-K27M mutation: A series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016. 26: 569-80
17. Takenaka S, Asano Y, Shinoda J, Nomura Y, Yonezawa S, Miwa K. Comparison of 11C-methionine, (11) C-choline, and (18)F-fluorodeoxyglucose-PET for distinguishing glioma recurrence from radiation necrosis. Neurol Med Chir (Tokyo). 2014. 54: 280-9
18. Tanaka M, Ino Y, Nakagawa K, Tago M, Todo T. High-dose conformal radiotherapy for supratentorial malignant glioma: A historical comparison. Lancet Oncol. 2005. 6: 953-60
19. van Zanten SE, Baugh J, Chaney B, de Jongh D, Aliaga ES, Barkhof F. Development of the SIOPE DIPG network, registry and imaging repository: A collaborative effort to optimize research into a rare and lethal disease. J Neuro Oncol. 2017. 132: 255-66