- Department of Neurological Surgery, Mount Sinai Hospital, New York City, United States.
- Department of Neurological Surgery, Mount Sinai Health System, New York City, United States.
Correspondence Address:
Tomoyoshi Shigematsu, Department of Neurological Surgery, Mount Sinai Health System, New York City, United States.
DOI:10.25259/SNI_1116_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Maximilian Jeremy Bazil1, Johanna T. Fifi2, Kurt A. Yaeger2, Reade A. De Leacy2, Christopher Paul Kellner2, Tomoyoshi Shigematsu2. Low-memory shape coils for intracranial aneurysm coiling: Initial and single-center experience with the i-ED coil. 21-Apr-2023;14:142
How to cite this URL: Maximilian Jeremy Bazil1, Johanna T. Fifi2, Kurt A. Yaeger2, Reade A. De Leacy2, Christopher Paul Kellner2, Tomoyoshi Shigematsu2. Low-memory shape coils for intracranial aneurysm coiling: Initial and single-center experience with the i-ED coil. 21-Apr-2023;14:142. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12281
Abstract
Background: Endovascular aneurysmal coiling is a preventative alternative to clipping to avoid aneurysmal rupture. In the literature and our own experience, some common coiling challenges which arise include: (1) microcatheter kickback, (2) detachment zone rigidity, (3) intrasaccular compartmentalization of coils on deployment, and (4) attainability of high-density and effective packing with as few coils as possible.
Methods: We retrospectively reviewed a consecutive case series of 15 intracranial aneurysm patients who received Kaneka i-ED Coils since their initial use in our practice (December 2020) till May 2022.
Results: Of the 14 saccular aneurysm patients treated with i-ED coils, 2/14 (14.3%) achieved a Raymond-Roy (RR) score of 3A (internal remnant), 4/14 (28.6%) achieved RR 2 (slight neck remnant) and 8/14 (57.1%) achieved RR 1. One MoyaMoya patient (5.9%) with a fusiform aneurysm also achieved a complete occlusion by parent artery takedown in this series. Aneurysm volumes ranged from 8.15 mm 3 to 315.5 mm 3 with an average packing density of 36.23% and a standard deviation 8.87%. At 30 days, most of our cohort scored a 0 on the modified Rankin scale (mRS) (11/15), with two patients scoring at an mRS score of 1, one at an mRS score of 4, and one at an mRS score of 6. Low-memory shape, coil cases achieved a significantly higher packing density (P P
Conclusion: Our initial experience with i-ED coils has shown that they are a feasible strategy in a number of differently sized and shaped aneurysms. While fewer coils overall were not a statistically significant finding in this study, the future studies with larger cohorts are necessary and in progress.
Keywords: Aneurysm, Coiling, Low-memory, i-ED
INTRODUCTION
Endovascular aneurysmal coiling is a preventative alternative to clipping to avoid aneurysmal rupture.[
MATERIALS AND METHODS
We retrospectively reviewed a consecutive case series of 15 intracranial aneurysm patients who received Kaneka i-ED Coils since their initial use in our practice (December 2020) and May 2022. While some of these cases used LMSCs in conjunction with more traditional coils, others relied entirely on LMSCs. We discuss the nature of the aneurysms treated, radiographic outcomes (packing density and Raymond-Roy, RR class), overall procedural outcomes at the end of treatment (complications and number of coils), and technical notes. Packing density was calculated by inputting the aneurysm dimensions, coil type, and coil length into the Angiosuite Calculator© application. Angiosuite Calculator© was selected due to its consideration of aneurysm size distribution/ shape as well as differences in pitch across a variety of coils. RR class was assessed by the neurointerventionalist (TS). Complications and other demographic features were pulled from the hospital’s EPIC system. This study was approved by the appropriate institutional review board (IRB) under IRB study-21-00749.
RESULTS
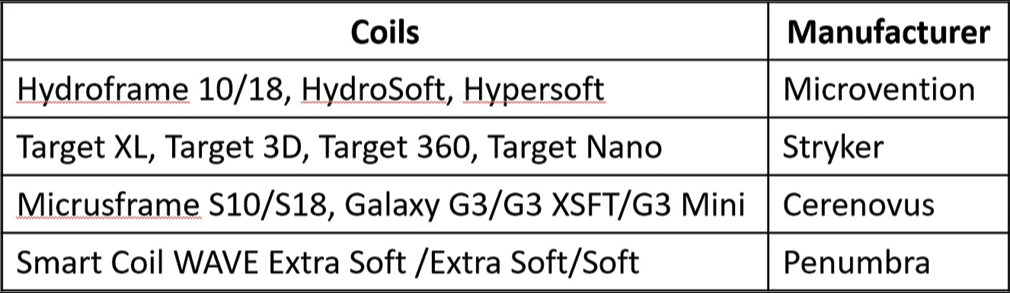
A specific selection criteria for use of Kaneka LMSCs were not employed during the study period; rather, if the coils were available and (at the interventionalist’s discretion) soft, LMSCs would be an effective choice for the aneurysm, they were used. Kaneka LMSCs were used in conjunction with other, more rigid, coils for framing, and/ or finishing purposes at the interventionalist’s discretion. Five cases were performed in conjunction with other coils in the initial deployment (HydroFrame 10 and HydroSoft 3D; Microvention; Aliso Viejo, California) (Target 360 Standard; Stryker; Kalamazoo, Michigan) (Galaxy G3; Cerenovus; Irvine, California), while the remaining ten used only Kaneka i-ED coils. The aneurysms in this series were distributed among the middle cerebral artery (MCA) (n = 4, 2 along the vessel, 1 off a large moya-moya collateral and another at the bifurcation), the posterior communicating artery (PComm) (n = 4), the anterior communicating artery (AComm) (n = 4), the anterior cerebral artery (n = 1 between A2 and A3 segments), and the basilar artery (n = 2, one along the side-wall and another at the tip). Most aneurysms were saccular (14/15) with a single fusiform case. Nine cases (60%) involved stent-assistance. Overall, 5/15 cases were performed immediately post-rupture, 1/15 was previously ruptured, and 9/15 were unruptured. Of the five ruptured cases, 4 (80%) were performed within 24 h of symptom onset. The exception to this was a 48-h delay in the patient’s appearance in the emergency room post symptom onset. The majority of the saccular aneurysms took on a traditional and oblate-spheroid shape (10/14) with three additional bilobed cases and a single side-wall aneurysm. Of the 14 saccular aneurysm patients treated with i-ED coils, 2/14 (14.3%) achieved a RR score of 3A (internal remnant), 4/14 (28.6%) achieved RR 2 (slight neck remnant) and 8/14 (57.1%) achieved RR 1 [
We compared the number of coils required for treatments using i-ED coils to total coils for treatment of the 50 most recent, non-i-ED coiling patients at our practice [Supplemental Figure 2: List of Non-i-ED Coils]. Cases were selected if they had not been previously embolized or clipped and only the first coiling treatment (if multiple) was assessed. Of the 50 cases reviewed, 92% (46/50) were saccular and 8% (4/50) were fusiform with volumes ranging between 14.1 mm3 and 1334.12 mm3. In this cohort, 11 patients scored a RR of 1, 19 scored a RR of 2, and 16 scored a RR of 3. A student’s one-tailed t-test did not achieve significance (P = 0.109) for the result of fewer coils used in i-ED cases (median: 4 coils, average: 3.87 coils SD: 1.41 coils) than others in our practice (median 4 coils, average 4.5 coils SD: 2.49 coils), but this finding should be re-investigated with a larger cohort of i-ED cases. We also compared the packing density at the end of each initial treatment for this cohort to the i-ED cohort. We found that cases which used i-ED coils (average: 36.23% packing density SD: 8.87%) achieved a significantly higher packing density (P < 0.01) than other cases in our practice (average: 24.8% packing density SD: 12.09%). The ratio of percent packing density achieved to coils used was significantly higher (P < 0.05) in the i-ED coil cases (average PD%/ Coils: 10.9 SD: 5.91) than in the non-i-ED cohort (average PD/Coils: 7.35 SD: 5.62).
Illustrative cases
We identified seven cases in our series with representative imaging of microcatheter kickback control and use for anatomically complex aneurysms which we describe below.
Kickback control
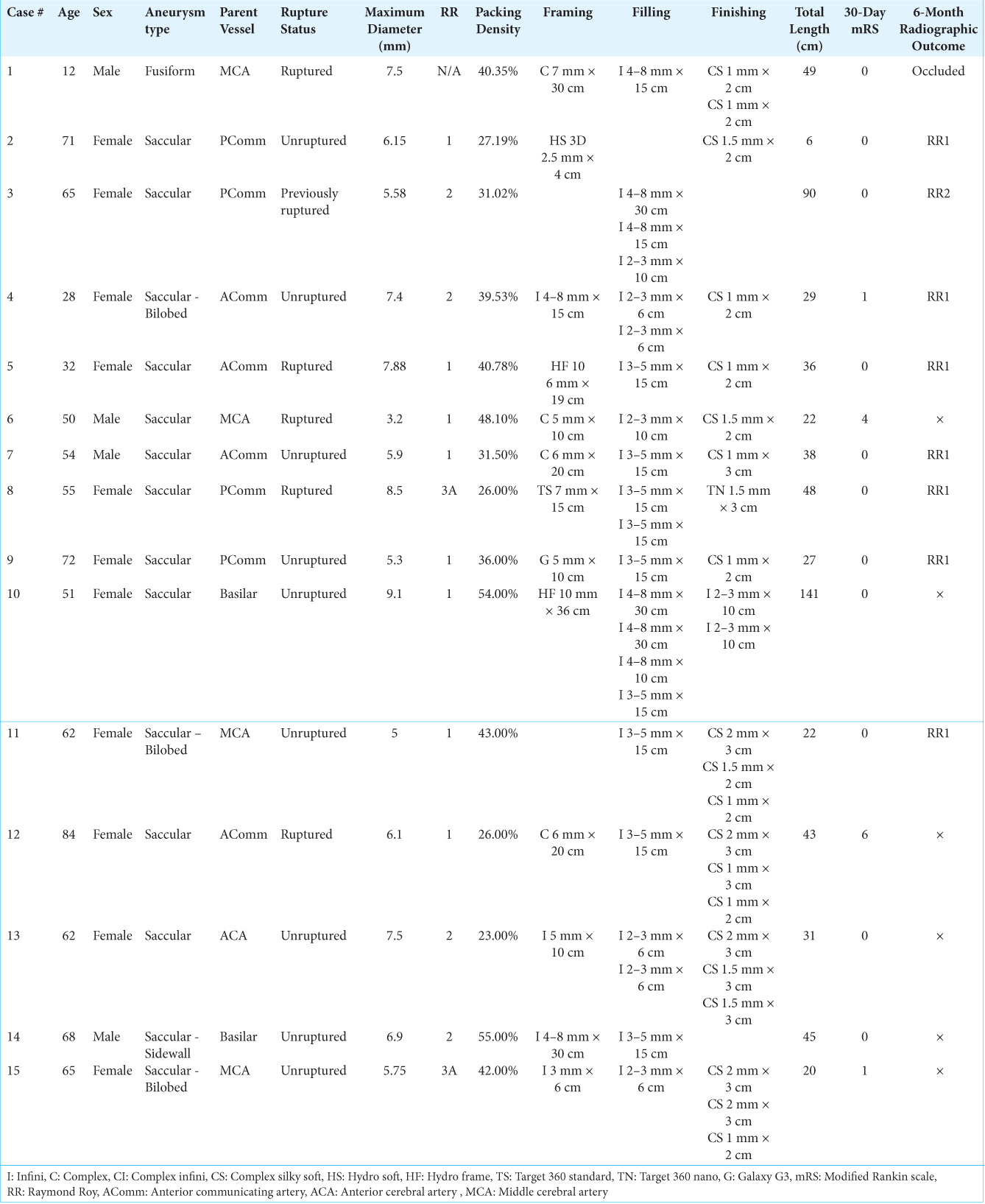
Two members of our series (Cases 1 and 2) demonstrated the LMSCs’ usefulness in controlling kickback when placing finishing coils into a densely packed environment [
Figure 1:
Kickback control with i-ED coils. A ruptured fusiform aneurysm on a collateralizing vessel (blue arrow) took a sharp turn from left M1 (yellow arrow) (a). The aneurysm was catheterized with a microcatheter and coiled densely with two i-ED coils (b). To achieve tighter packing at the orifice of the aneurysm where the microcatheter was likely to be kicked out, two small i-ED SilkySoft coils were added without microcatheter kickback (c). During the balloon-assisted coiling of a small, left PComm (yellow) aneurysm (blue) (d), after placing one HydroSoft coil (e), another i-ED SilkySoft coil was added to achieve tighter packing of the aneurysm without interventionalist-noted kickback of the microcatheter (f).
Irregular aneurysms
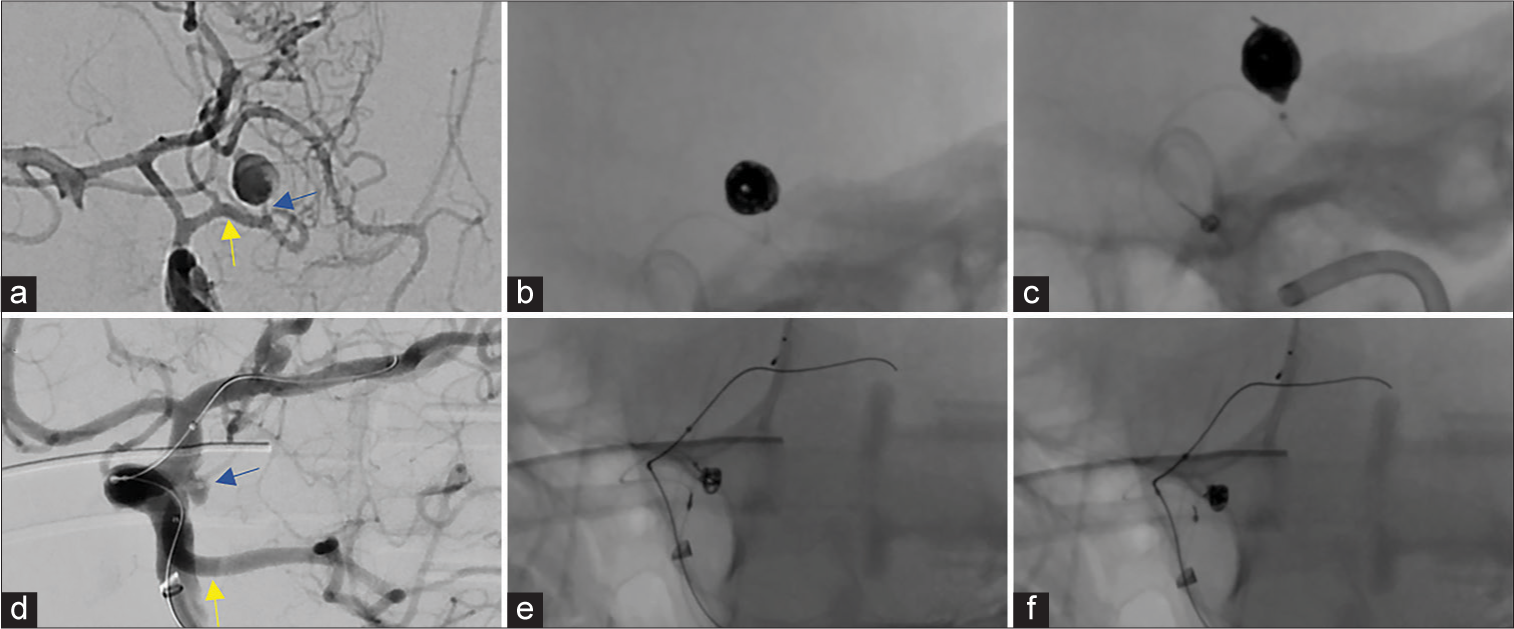
Two irregular aneurysm cases (Cases 3, 4) were selected, based on the experience of the attending physician, to outline in greater detail [
Figure 2:
Anatomically complex aneurysm coiling with i-ED Coils: A previously coiled right posterior communicating artery aneurysm with an uncertain neck diameter (a) experienced coil compaction and required stent-assisted coiling. Coiling was performed with low-memory shaped i-ED Infini coils which filled the unclear residual space (b). The patient achieved a RR2 obliteration of the residual aneurysm (c). In the irregularly shaped, bi-lobulated, anterior communicating artery aneurysm (d), the low-memory shaped i-ED Infini coil expanded into both lobes quite well to create a durable frame in a stent-assisted coiling procedure (e). With two filling coils and a finishing coil, the aneurysm was completely obliterated (f).
Minimal coil usage
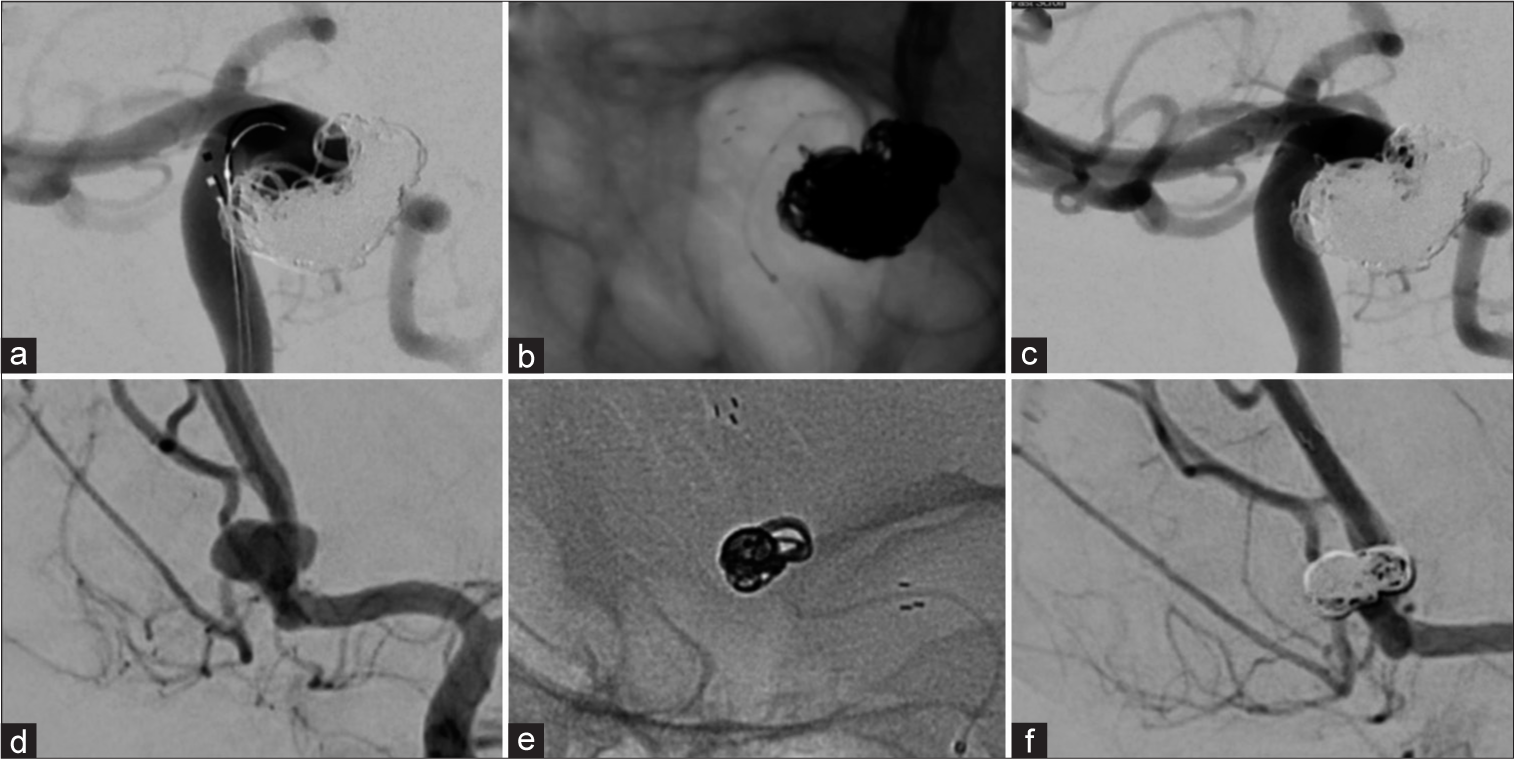
Finally, we present Case 5, Case 6 and Case 7 which received just one low-memory shaped Complex Infini coil as a filling coil, one as a finishing coil, with a single coil from either i-ED or the Hydroframe 10 as a framing coil. These cases achieved a high packing density in each case (31.0%, 33.0% and 31.5%, respectively;
Figure 3:
Efficient packing with Kaneka i-ED coils. Three aneurysms (an anterior communicating artery [AComm] aneurysm [a], a left MCA bifurcation aneurysm [f], and another AComm aneurysm [k]) were each coiled using one framing coil (b, g, l), one filling i-ED low memory shaped Infini coil (c, h, m) and one small finishing i-ED SilkySoft coil (d, i, n). Favorable angiographic results were achieved (RR = 1 [e], RR = 2 [j], and RR = 1 [o]).
DISCUSSION
Our practice examined the initial experience of Kaneka i-ED coils in the United States in a series of 15 patients treated either entirely with Kaneka coils or in combination with other coiling products. The i-ED coil series is distinct from Kaneka “ED” coils which also demonstrated highly effective coiling in a 92-member cohort and a 345-member cohort in Japan.[
The i-ED Infini and Complex Infini coils are low-memory shape coils with a relatively long length (between 6 cm and 50 cm). Traditional coils have a pre-shaped structure which may create loops within the aneurysm rather than along the edges or on the wall. This may further cause microcatheter kickback when attempting to deploy additional coils or may create strain on the overall aneurysm structure. We have found that in certain aneurysms, dense packing is achievable with as little as two or even one coil. The impact of this technology is especially visible in cases of irregular anatomy, as we have observed the Infini coils to fill up any space they occupy regardless of the shape. We believe that this feature makes the coils far less likely to compartmentalize. Regarding compartmentalization, LMSCs extrude to occupy (“find”) whatever space is available, laying flush against wall of the aneurysm and avoiding formation of internal loops/shapes. Thus, we expected i-ED Infini coils to be relatively versatile as the main consideration is length rather than coil diameter, especially for the filling coils. This helps to simplify coil selection intraprocedurally.
We find that the coils packed and detached as expected without issue. The detachment system of the Kaneka i-ED coils is a monopolar system, based on placement of a hypodermic needle as the ground for an “electro-detach generator” which sends current through the main deployment wire to melt the PVC that attaches the coils to the catheter. While this system is rather outdated, we believe that the softness of the detachable component makes up for this. The softness of both the detachment and the coils themselves allow for excellent microcatheter kickback control. In this way, the SilkySoft technology introduced in the i-ED coil series has shown promise to become a highly effective treatment. In our initial experience, there was no issue in i-ED Infini coils’ compatibility with any framing coil on the market. A notable issue we have yet to experience with the i-ED coils, but believe should be examined more closely, is the risk for coil compaction. The softness of the coil may pose an increased risk of turbulent blood flow, undoing the structure of the coils which may lead to rupture and/or the necessity for retreatment. The softness of the coils may be made up for by achieving a high packing density; the literature has shown that achieving higher packing density is the key to fewer instances of recanalization,[
CONCLUSION
Our initial experience with i-ED coils has demonstrated their feasibility in aneurysm treatment and a trend, though not significant, of using fewer coils to achieve a higher packing density. Moreover, the i-ED coils seem to simplify the coiling procedure by allowing for selection of filling coils based only on the length of the low-memory shaped Infini coils and by preventing microcatheter kickback on deployment due to the softness of the coils. We are not able to effectively assess safety differences between LMSCs and traditional coils at this time. The i-ED coils have shown preliminary success in our cohort. We are currently performing a larger case series to investigate these findings more rigorously.
Declaration of patient consent
Institutional review board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTAL FIGURES
References
1. Bendok BR, Abi-Aad KR, Ward JD, Kniss JF, Kwasny MJ, Rahme RJ. The hydrogel endovascular aneurysm treatment trial (HEAT): A randomized controlled trial of the second-generation hydrogel coil. Neurosurgery. 2020. 86: 615-24
2. Cottier JP, Pasco A, Gallas S, Gabrillargues J, Cognard C, Drouineau J. Utility of balloon-assisted Guglielmi detachable coiling in the treatment of 49 cerebral aneurysms: A retrospective, multicenter study. AJNR Am J Neuroradiol. 2001. 22: 345-51
3. Currie S, Mankad K, Goddard A. Endovascular treatment of intracranial aneurysms: Review of current practice. Postgrad Med J. 2011. 87: 41-50
4. Fukutome K, Aketa S, Fukumori J, Mitsui T, Nakajima T, Hayami H. Cerebral vasospasm after coil embolization for unruptured anterior communicating artery aneurysm: Illustrative case. J Neurosurg Case Lessons. 2022. 3: CASE2288
5. Gross BA, Smith ER, Scott RM, Orbach DB. Intracranial aneurysms in the youngest patients: Characteristics and treatment challenges. Pediatr Neurosurg. 2015. 50: 18-25
6. Harada K, Morioka J. Initial experience with an extremely soft bare platinum coil, ED coil-10 Extra Soft, for endovascular treatment of cerebral aneurysms. J Neurointerv Surg. 2013. 5: 577-81
7. Imahori T, Mizobe T, Fujinaka T, Miura S, Sugihara M, Aihara H. An aneurysm at the origin of a duplicated middle cerebral artery treated by stent-assisted coiling using the “Wrapped-Candy” Low-Profile Visualized Intraluminal Support (LVIS) technique: A technical case report and review of the literature. World Neurosurg. 2020. 143: 353-9
8. Ishibashi T, Maruyama F, Kan I. Four-dimensional digital subtraction angiography for exploration of intraosseous arteriovenous fistula in the sphenoid bone. Surg Neurol Int. 2021. 12: 85
9. Kimura S, Yagi R, Kishi F, Ogawa D, Yamada K, Taniguchi H. Subarachnoid hemorrhage due to ruptured cerebral aneurysm at the distal part of anterior inferior cerebellar artery-posterior inferior cerebellar artery variant after γ-knife irradiation. Interdiscipl Neurosurg. 2022. 29: 101533
10. Mascitelli JR, Oermann EK, De Leacy RA, Moyle H, Mocco J, Patel AB. Predictors of treatment failure following coil embolization of intracranial aneurysms. J Clin Neurosci. 2015. 22: 1275-81
11. Mascitelli JR, Moyle H, Oermann EK, Polykarpou MF, Patel AA, Doshi AH. An update to the Raymond-Roy occlusion classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg. 2015. 7: 496-502
12. Mizutani K, Akiyama T, Tomita H, Toda M. Role of endovascular treatment for ruptured aneurysms involving the anterior spinal artery at the craniocervical junction. J Neuroradiol. 2022. 50: 44-9
13. Molyneux A, Kerr R. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomized trial. J Stroke Cerebrovasc Dis. 2002. 11: 304-14
14. Parkinson RJ, Eddleman CS, Batjer HH, Bendok BR. Giant intracranial aneurysms: Endovascular challenges. Neurosurgery. 2006. 59: S3-103
15. Sadato A, Hayakawa M, Adachi K, Hirose Y. Relationship between the volume rate of Ed coil (Ed Ratio) and packing density in endosaccular embolization of cerebral aneurysms. Asian J Neurosurg. 2018. 13: 619-25
16. Wang F, Chen X, Wang Y, Bai P, Wang HZ, Sun T. Stent-assisted coiling and balloon-assisted coiling in the management of intracranial aneurysms: A systematic review and meta-analysis. J Neurol Sci. 2016. 364: 160-6