- Department of Surgery, Prince of Wales Hospital, Shatin, Hong Kong.
Correspondence Address:
Tat Ming Danny Chan, Department of Surgery, Prince of Wales Hospital, Shatin, Hong Kong.
DOI:10.25259/SNI_795_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lok Wa Laura Leung, Yuen Chung David Chan, Tat Ming Danny Chan. Lumbar epidural blood patch: An effective treatment for intracranial hypotension. 11-Nov-2022;13:517
How to cite this URL: Lok Wa Laura Leung, Yuen Chung David Chan, Tat Ming Danny Chan. Lumbar epidural blood patch: An effective treatment for intracranial hypotension. 11-Nov-2022;13:517. Available from: https://surgicalneurologyint.com/surgicalint-articles/11999/
Abstract
Background: The literature has demonstrated the efficacy of lumbar epidural blood patch (LEBP) in the management of spontaneous intracranial hypotension (SIH). However, the underlying pathophysiology of such management remains unclear. In this study, we aim to evaluate the utility of LEBP injections in the management of SIH and develop a potential management algorithm used in the triage and management of SIH patients.
Methods: We retrospectively examined the clinical case notes of 14 patients with SIH (age: 25–69 years) who were managed with LEBP injections during the year of 2016–2021. We evaluated the presenting symptoms of each selected patient and radiological findings as well as treatment outcomes. Our aim is to evaluate the effectiveness of LEBP in the treatment of SIH patients through follow-up clinical and imaging assessment.
Results: About 93% of patients describe the presence of headache at presentation, while 43% describe it as being of an orthostatic nature. All patients demonstrated typical findings on magnetic resonance imaging brain. Treatment success assessed through symptomatic improvement and radiological resolution was found in 85% of our patients at a 2-month interval.
Conclusion: LEBP injection is an effective method of management in patients with a diagnosis of SIH. It should be considered in all SIH patients irrespective of whether a “dural leak” can be localized through radiological investigations.
Keywords: Cerebrospinal fluid leakage, Epidural blood patch, Postural headache, Spontaneous intracranial hypotension
INTRODUCTION
Spontaneous intracranial hypotension (SIH) continues to pose a diagnostic and therapeutic challenge to practicing clinicians.[
Clinically, the condition is primarily described as a postural headache,[
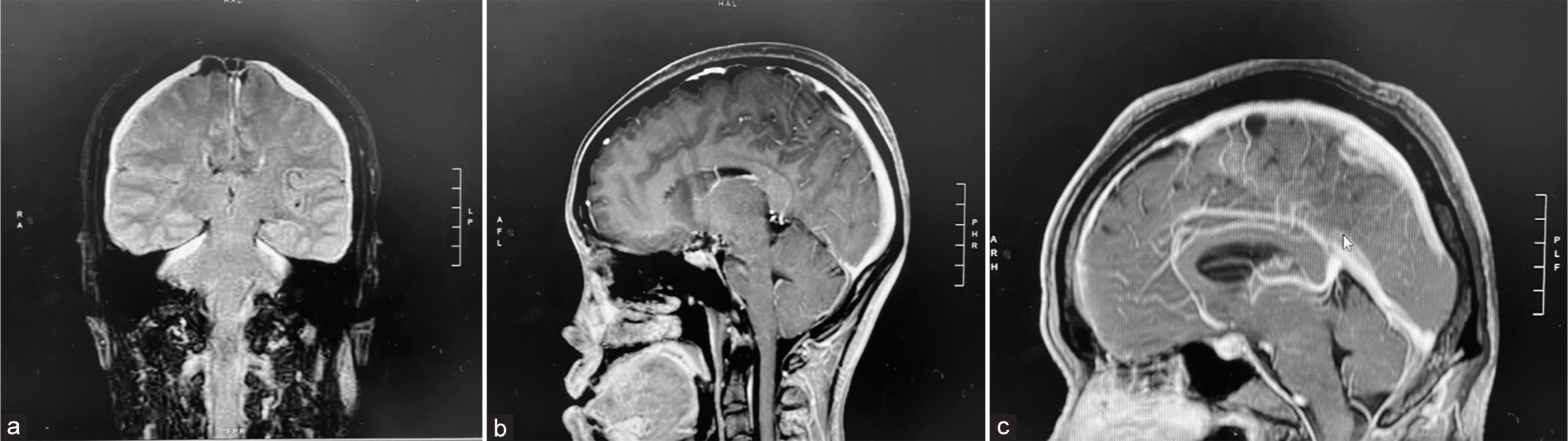
Radiologically, pathognomonic magnetic resonance imaging (MRI) findings of the brain include venous engorgement, diffuse dural thickening, and intracranial pachymeningeal gadolinium enhancement, reactive hyperemia of the pituitary gland, subdural collections, and hematomas, and brain sagging.[
Current practice in the management of patients with SIH includes both conservative and surgical modalities. In general, patients are advised to begin with conservative methods, where many improve without any further intervention. Other methods of management include rest with Trendelenburg positioning, rehydration with oral and intravenous fluids, caffeine intake, and abdominal binder.[
Our retrospective study aims to examine the presenting symptoms of SIH and to validate the use of lumbar epidural blood patch (LEBP) in the treatment of SIH within a Chinese-based cohort.
MATERIALS AND METHODS
Patients
We retrospectively identified all patients who were given an EBP between January 2016 and December 2021 at the Prince of Wales Hospital, Hong Kong. All patients were aged above 18 years old and received a LEBP based on the suspicion of a diagnosis of SIH. Cases must have received a preinjection MRI brain. Obstetrics and gynecology patients who received underwent spinal/epidural anesthesia and received LEBP injections as treatment for intracranial hypotension were excluded from the study. In total, 14 patients met the above inclusion criteria (six females, eight males, mean age: 46.5 years, range: 25–69 years). Cases were reviewed through the analysis of clinical notes, where details including radiological images, follow-up consultation notes as well as the effectiveness of treatment were subjectively evaluated. The study protocol was approved by the CUHK-PWH CREC (CREC No. 2021–527).
Radiological findings
MRI findings of the brain were considered positive when any of the following findings were identified within the radiological report: Subdural hygroma, enhancement of pachymeninges, engorged veins, pituitary hyperemia, and sagging of the brain. Classical MRI brain findings of SIH are shown in
Delivery of LEBP
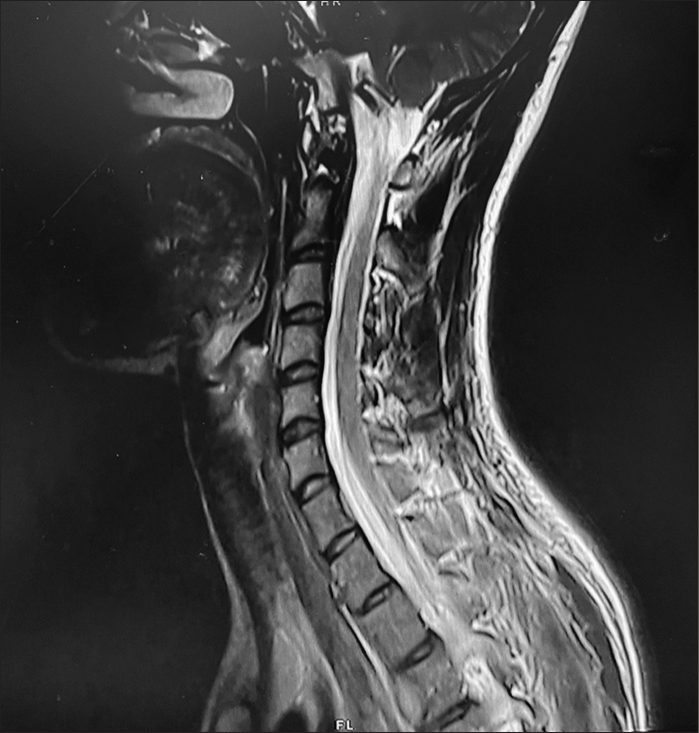
The LEBP was delivered by a team of neurosurgeons and/or anesthetists, through a midline approach at the lumbar spine region (L2–L5). 20 ml of autologous blood was taken from the patient’s arm and injected into the patient’s spinal region, and patients were monitored postoperatively both through inpatient and outpatient care.
Treatment success
We aimed to measure the presence and degree of treatment success within our patients after LEBP injections. Treatment success was evaluated by two means, first the alleviation of symptoms defined as any symptomatic improvement noted at the first postinjection outpatient follow-up with the neurosurgical team and second through radiological resolution. Symptomatic improvement was reported as resolution (total cessation with no further episodes), moderate (partial cessation +/- minimal further episodes), and worsened (worsening of symptoms). Symptoms such as headache, orthostatic headache, nausea, vomiting, meningism, tinnitus, and dizziness were accounted for. Improvements in characteristic radiological features, that is, resolution of subdural hygromas on CT or resolution of classic MRI findings were also recorded. All results obtained within a 2-month interval post-LEBP injection were taken into account of.
RESULTS
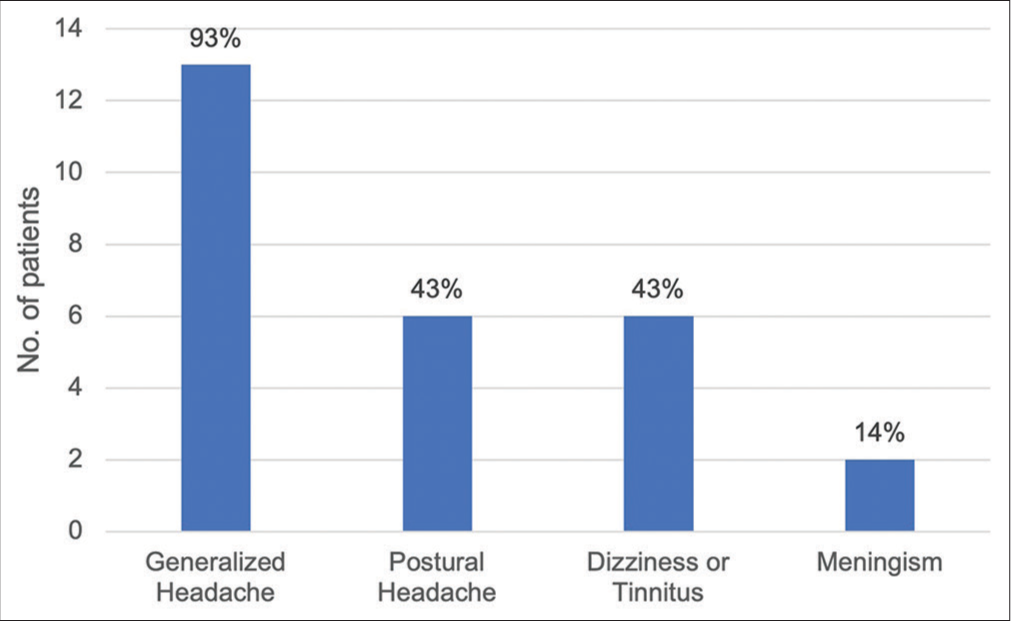
We first assessed the clinical manifestations of all patients who were diagnosed with SIH. About 43% of patients described the features of a postural headache, while 93% of patients mentioned the presence of a generalized headache. About 50% of patients reported symptoms of nausea and vomiting, 43% had dizziness or tinnitus, and 14% reported features of meningism [
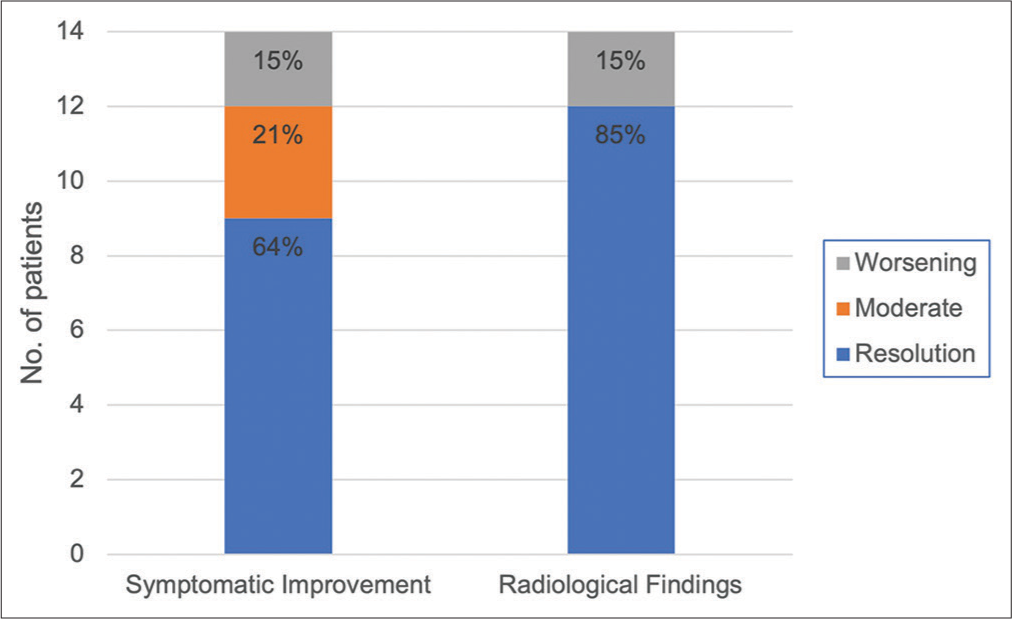
The mean admission duration to the hospital was 16 days, where 11 patients were admitted under the neurosurgery service and three patients under the medical service. All patients received one LEBP injection, with a median volume of 26 ml (range: 20–30ml). The LEBP injections were most often completed at the spinal levels of L3/L4 (50%), followed by L2/L3 (36%) and L4/L5 (14%). Five patients received burr hole drainages for their subdural hematomas, while one attempted aggressive intravenous fluid replacement for 2 weeks before further management. In terms of treatment response to LEBP, patients were seen for a follow-up duration of between 2 months and 2 years. Symptomatic improvement was achieved in 85% of our patients, with 64% demonstrating complete resolution of symptoms and 21% attaining moderate improvement in symptoms. About 15% complained of further worsening in symptoms [
Figure 4:
Chart illustrating post lumbar epidural blood patch treatment success through symptomatic improvement and radiological findings. Symptomatic improvement was reported as resolution (total cessation with no further episodes), moderate (partial cessation +/- minimal further episodes), and worsened (worsening of symptoms). Improvements in characteristic radiological features, that is, resolution of subdural hygromas on CT and/or resolution of classic MRI findings.
DISCUSSION
In this retrospective study, we evaluated the use of LEBP in the management of patients with SIH, who were initially diagnosed with supporting clinical and radiological evidence. Existing literature recommends that all symptomatic SIH patients receive an EBP injection, with an existing efficacy rate ranging between 30 and 60%.[
Our results show that one LEBP injection led to the resolution of symptoms and radiological features within 85% of patients [
Pathophysiology of SIH
Despite the poorly understood pathophysiology of SIH, two general schools of thought are found within the existing literature. Classically, clinicians believe that a spontaneous or traumatic dural leak and its associated CSF hypovolemia are responsible for the presentation of SIH. Yet, existing literature fails to explain why certain patients respond to LEBP treatment despite the lack of CSF leak. This has led to the development of an alternative hypothesis, suggesting that SIH is associated with the over drainage of venous blood from the epidural spinal vein network of the brain.[
Dangers of a targeted EBP
In patients with an identifiable dural leak on MRI myelogram, studies have indicated that a targeted EBP was more advantageous in improving patient outcome relative to a blind EBP.[
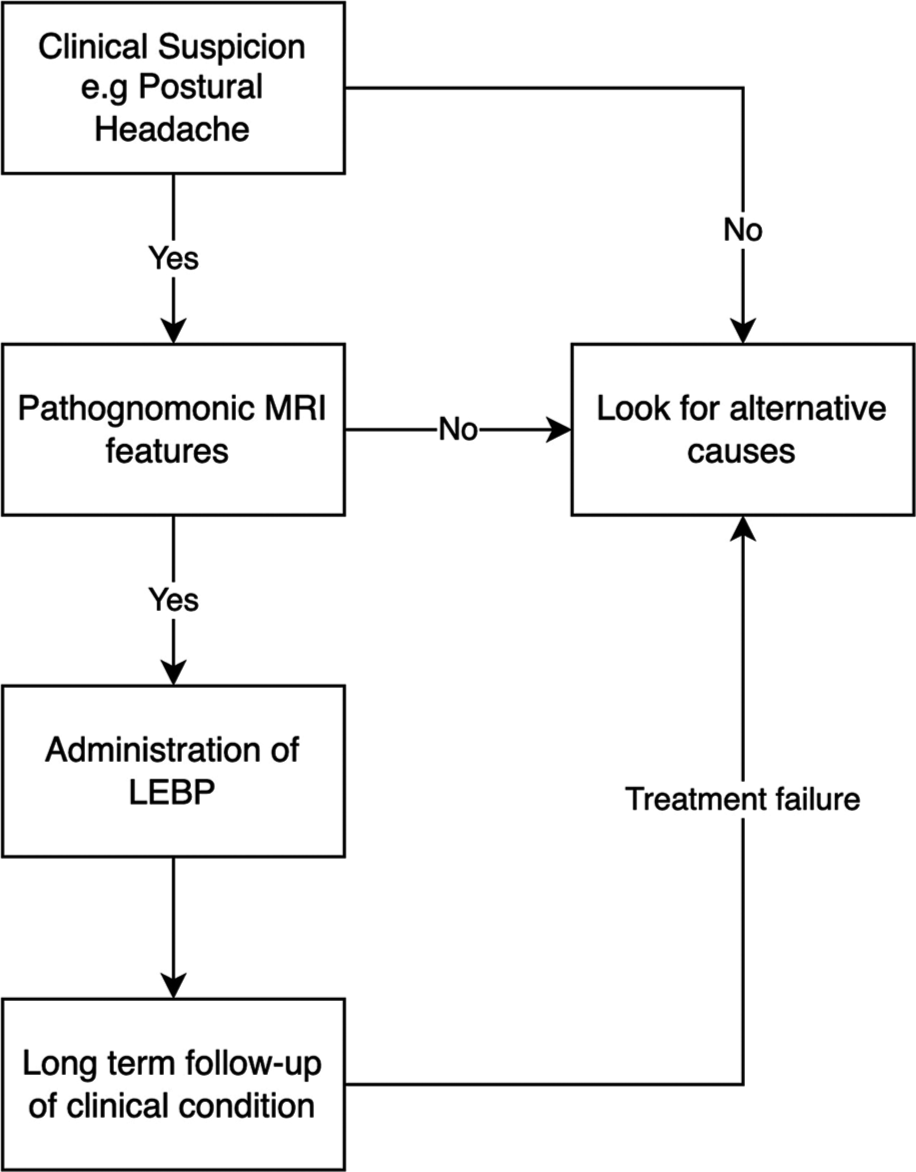
Proposed management algorithm
Characteristically, the presence of a generalized headache with a postural component raises our clinical suspicion for a potential case of SIH. Within our cohort, 90% of patients described the presence of a generalized headache, though only 43% of patients mentioned a postural or orthostatic component to the headache. Other suggestive features include tinnitus and/or dizziness, nausea, and/or vomiting. About 23% of patients also recalled some form of head trauma before the event. Very often, this indicates that clinical features may not be particularly sensitive for the detection of SIH. However, we found that an MRI brain was crucial for the diagnosis of SIH. Typical radiological features of SIH were seen in all of our patients, indicating that radiological evidence may indeed be the most reliable method of diagnosis for SIH. MRI myelogram can be considered when needed and available, although the added information may not necessarily benefit the management outcome of patients. In all patients diagnosed with SIH, we recommend the first LEBP injection between the levels of L2–L5, with target sites aimed past the conus medullaris. Directed by either the neurosurgical or anesthetic team, 20–30 ml of autologous blood will be inserted into the epidural space, or until the patient complains of a headache. In patients with evidence of an identifiable CSF leak on radiological investigations, a targeted EBP may be considered as a second-line treatment. The previous literature has supported the evidence of targeted EBP injections, although not without its own risk. Postoperatively, the duration and frequency of follow-up will depend on the initial treatment response, and further investigation and treatment may be warranted in those who fail to respond to a LEBP. The proposed algorithm is summarized in
Examining treatment failures in LEBP
Despite the success of LEBP injections in the symptomatic improvement of patients, 15% of our sampled population also demonstrated worsening of symptoms. Interestingly, both patients were found to receive an LEBP injection after first burr hole drainage of their chronic subdural hematomas. Both patients were also found to develop worsening signs and symptoms, along with radiological progression of their chronic subdural hematomas on follow-up imaging. Repeated burr hole drainages were then completed, with long-term follow-ups demonstrating improvement in clinical condition. Both patients were found to demonstrate deterioration of condition within a 2-month follow-up period. This reinforces the importance of long-term follow-up after administration of LEBP, through both in-person clinical evaluation as well as collection of radiological evidence.
CONCLUSION
We believe that LEBP is indeed a successful and effective way of managing patients who have a suspected diagnosis of SIH. Our results support that LEBP is an integral part in the reversal of epidural hypotension, allowing for a change in CSF-hematic gradient, and therefore improvement in the condition of SIH. Therefore, we believe that the recipients of LEBP should not be limited to those who have an identifiable dural leak as other existing literature may indicate.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments and disclosures
This work is supported by the Prince of Wales Hospital and Chinese University of Hong Kong. We confirm that this work is original and has not been published or under consideration for publication elsewhere. We have no conflicts of interest to disclose.
References
1. Ahn C, Lee E, Lee JW, Chee CG, Kang Y, Kang HS. Two-site blind epidural blood patch versus targeted epidural blood patch in spontaneous intracranial hypotension. J Clin Neurosci. 2019. 62: 147-54
2. Beck J, Häni L, Ulrich CT, Fung C, Jesse CM, Piechowiak E. Diagnostic challenges and therapeutic possibilities in spontaneous intracranial hypotension. Clin Transl Neurosci. 2018. 2: 2514183
3. Beckhardt RN, Setzen M, Carras R. Primary spontaneous cerebrospinal fluid rhinorrhea. Otolaryngol Head Neck Surg. 1991. 104: 425-32
4. Chen HH, Huang CI, Hseu SS, Lirng JF. Bilateral subdural hematomas caused by spontaneous intracranial hypotension. J Chin Med Assoc. 2008. 71: 147-51
5. Cho KI, Moon HS, Jeon HJ, Park K, Kong DS. Spontaneous intracranial hypotension: Efficacy of radiologic targeting vs blind blood patch. Neurology. 2011. 76: 1139-44
6. Davidson B, Nassiri F, Mansouri A, Badhiwala JH, Witiw CD, Shamji MF. Spontaneous intracranial hypotension: A review and introduction of an algorithm for management. World Neurosurg. 2017. 101: 343-9
7. Davies MJ, Davies MA, Sharpe R, Cordato D, Schwartz R. Epidural blood patch as a diagnostic and therapeutic intervention in spontaneous intracranial hypotension: A novel approach to management. World Neurosurg. 2020. 137: e242-50
8. Erkulvrawatr S, El Gammal T, Hawkins J, Green JB, Srinivasan G. Intrathoracic meningoceles and neurofibromatosis. Arch Neurol. 1979. 36: 557-9
9. Ferrante E, Trimboli M, Rubino F. Spontaneous intracranial hypotension: Review and expert opinion. Acta Neurol Belg. 2020. 120: 9-18
10. Fontaine N, Charpiot A, Debry C, Gentine A. A case of spontaneous intracranial hypotension: From Ménière-like syndrome to cerebral involvement. Eur Ann Otorhinolaryngol Head Neck Dis. 2012. 129: 153-6
11. Franzini A, Messina G, Nazzi V, Mea E, Leone M, Chiapparini L. Spontaneous intracranial hypotension syndrome: A novel speculative physiopathological hypothesis and a novel patch method in a series of 28 consecutive patients. J Neurosurg. 2010. 112: 300-6
12. Franzini A, Zekaj E, Messina G, Mea E, Broggi G. Intracranial spontaneous hypotension associated with CSF cervical leakage successfully treated by lumbar epidural blood patch. Acta Neurochir (Wien). 2010. 152: 1997-9
13. Guan J, Couldwell WT, Taussky P. Intracranial hypotension as a complication of lumbar puncture prior to elective aneurysm clipping. Surg Neurol Int. 2014. 5: S427-9
14. He FF, Li L, Liu MJ, Zhong TD, Zhang QW, Fang XM. Targeted epidural blood patch treatment for refractory spontaneous intracranial hypotension in China. J Neurol Surg B Skull Base. 2018. 79: 217-23
15. Jones MR, Shlobin NA, Dahdaleh NS. Spontaneous spinal cerebrospinal fluid leak: Review and management algorithm. World Neurosurg. 2021. 150: 133-9
16. Kranz PG, Luetmer PH, Diehn FE, Amrhein TJ, Tanpitukpongse TP, Gray L. Myelographic techniques for the detection of spinal CSF leaks in spontaneous intracranial hypotension. AJR Am J Roentgenol. 2016. 206: 8-19
17. Lee GH, Kim J, Kim HW, Cho JW. Comparisons of clinical characteristics, brain MRI findings, and responses to epidural blood patch between spontaneous intracranial hypotension and post-dural puncture headache: Retrospective study. BMC Neurol. 2021. 21: 253
18. Lee GK, Abrigo JM, Cheung TC, Siu DY, Chan DT. Spontaneous intracranial hypotension: Improving recognition and treatment strategies in the local setting. Hong Kong Med J. 2014. 20: 537-40
19. Levine DN, Rapalino O. The pathophysiology of lumbar puncture headache. J Neurol Sci. 2001. 192: 1-8
20. Lin JP, Zhang SD, He FF, Liu MJ, Ma XX. The status of diagnosis and treatment to intracranial hypotension, including SIH. J Headache Pain. 2017. 18: 4
21. Madsen SA, Fomsgaard JS, Jensen R. Epidural blood patch for refractory low CSF pressure headache: A pilot study. J Headache Pain. 2011. 12: 453-7
22. Mokri B. Cerebrospinal fluid volume depletion and its emerging clinical/imaging syndromes. Neurosurg Focus. 2000. 9: e6
23. Mokri B. Headaches caused by decreased intracranial pressure: Diagnosis and management. Curr Opin Neurol. 2003. 16: 319-26
24. Mokri B. Spontaneous low pressure, low CSF volume headaches: Spontaneous CSF leaks. Headache. 2013. 53: 1034-53
25. Perez-Vega C, Robles-Lomelin P, Robles-Lomelin I, Garcia Navarro V. Spontaneous intracranial hypotension: Key features for a frequently misdiagnosed disorder. Neurol Sci. 2020. 41: 2433-41
26. Rahman M, Bidari SS, Quisling RG, Friedman WA. Spontaneous intracranial hypotension: Dilemmas in diagnosis. Neurosurgery. 2011. 69: 4-14
27. Rettenmaier LA, Park BJ, Holland MT, Hamade YJ, Garg S, Rastogi R. Value of targeted epidural blood patch and management of subdural hematoma in spontaneous intracranial hypotension: Case report and review of the literature. World Neurosurg. 2017. 97: 27-38
28. Schievink WI, Smith KA. Nonpositional headache caused by spontaneous intracranial hypotension. Neurology. 1998. 51: 1768-9
29. Schievink WI, Torres VE. Spinal meningeal diverticula in autosomal dominant polycystic kidney disease. Lancet. 1997. 349: 1223-4
30. Schievink WI, Wijdicks EF, Meyer FB, Sonntag VK. Spontaneous intracranial hypotension mimicking aneurysmal subarachnoid hemorrhage. Neurosurgery. 2001. 48: 513-6
31. Schievink WI. Misdiagnosis of spontaneous intracranial hypotension. Arch Neurol. 2003. 60: 1713-8
32. Schievink WI, Gordon OK, Tourje J. Connective tissue disorders with spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension: A prospective study. Neurosurgery. 2004. 54: 65-70
33. Smith KA. Spontaneous intracranial hypotension: Targeted or blind blood patch. J Clin Neurosci. 2016. 25: 10-2
34. Urbach H. Intracranial hypotension: Clinical presentation, imaging findings, and imaging-guided therapy. Curr Opin Neurol. 2014. 27: 414-24