- Department of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India
- Department of Neurosurgery, National Institute of Mental Health and Neurosciences, Bengaluru, Karnataka, India
- Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Ankur Kapoor
Department of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India
DOI:10.4103/2152-7806.178569
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Singla N, Kapoor A, Savardekar A, Radotra BD, Chatterjee D, Gupta SK. Malignant cerebellar peduncle lesions - rapid progression and poor outcome. Surg Neurol Int 11-Mar-2016;7:
How to cite this URL: Singla N, Kapoor A, Savardekar A, Radotra BD, Chatterjee D, Gupta SK. Malignant cerebellar peduncle lesions - rapid progression and poor outcome. Surg Neurol Int 11-Mar-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/malignant-cerebellar-peduncle-lesions-%e2%80%91-rapid-progression-and-poor-outcome/
Abstract
Background:Tumors arising from cerebellar peduncle are extremely rare and behave aggressively. The inclusion of these into either cerebellar or brainstem gliomas is contentious.
Case Description:We performed clinicopathological review of three patients treated at our institute and surveyed the literature for previous such reported cases. Mean duration of symptoms in our patients was 2 weeks. Subtotal tumor resection was performed in two patients while the third underwent stereotactic biopsy followed by chemoradiotherapy. Histopathology revealed glioblastoma in initial two patients and medulloblastoma Grade IV in the third. The two patients who underwent surgical excision succumbed to the illness within 2 days and a month, respectively.
Conclusion:Malignant cerebellar peduncular lesions have poor overall survival despite surgical debulking. It is not confirmed whether these tumors should be considered as cerebellar lesions or brainstem gliomas due to aggressive clinical behavior, and so the ideal line of management is not yet known.
Keywords: Glioblastoma cerebellar peduncle, malignant peduncular lesions, outcome cerebellar lesions
INTRODUCTION
Primary cerebellar peduncle lesions are defined as the ones that arise directly from the peduncle and spread to involve the neighboring cerebellum and brainstem vital areas. Malignant lesions at this location are rare. It is a dilemma whether they behave as cerebellar tumors or as brainstem tumors. This ignites controversy of whether radical surgery is beneficial or histological diagnosis followed by radiotherapy should be attempted. There is scarce literature on the same and prognosis remains dismal despite surgery and radiotherapy. These tumors are known for their low overall survival (OS) and early recurrence.[
CASE REPORTS
Case 1
A 10-year-old male presented with complaints of gait disturbance and vomiting for 2 weeks. On examination, he was drowsy and had left sided cerebellar signs and a weak gag reflex on left side. Magnetic resonance imaging (MRI) [
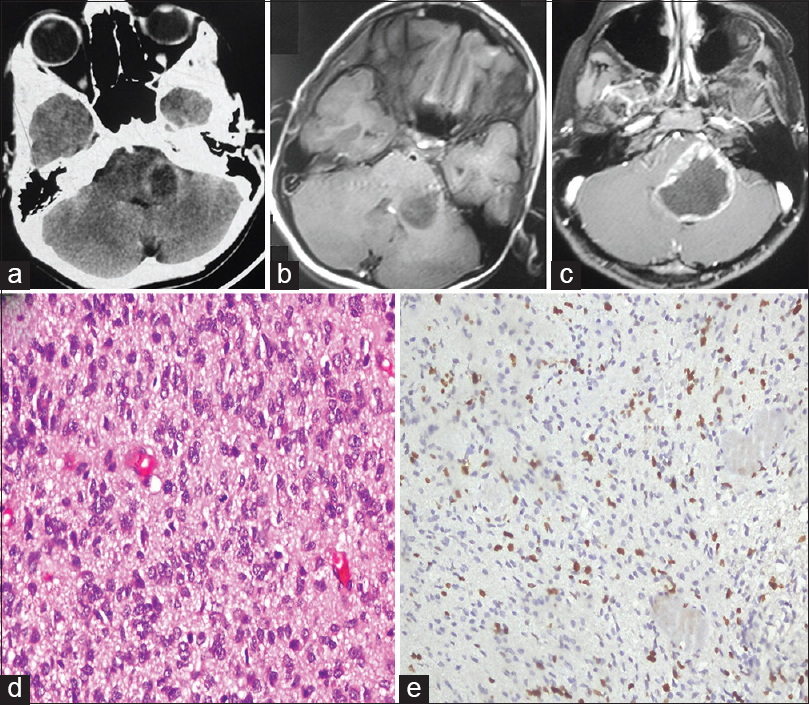
Figure 1
(a) Noncontrast computed tomography (axial) showing hypodense lesion at left cerebellar peduncle. (b) T1-weighted contrast axial image showing nonenhancing lesion before first surgery. (c) Axial T1-weighted contrast image at recurrence showing solid-cystic lesion at peduncle. (d) Photomicrograph showing tumor cells with nuclear pleomorphism, high mitotic activity (H and E, ×40). (e) Ki-67 immunostain showing very high proliferation index (IP, ×40)
Case 2
A 9-year-old female presented with complaint of diplopia on far vision and facial deviation for 1 week. Examination revealed gait ataxia, left gaze, facial paresis, and subtle left cerebellar signs. MRI [
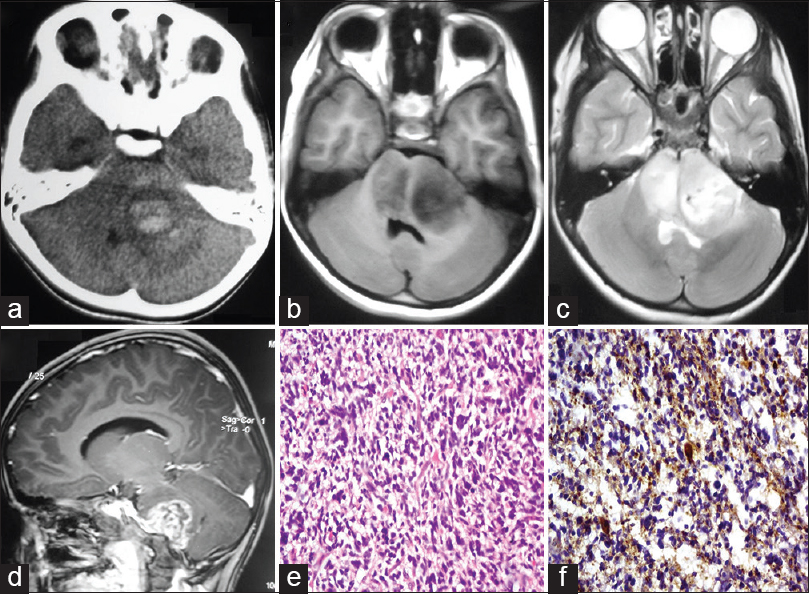
Figure 2
(a) Noncontrast computed tomography (axial) showing hemorrhagic lesion at left cerebellar peduncle. (b-d) Axial T1-weighted, axial T2-weighted, and sagittal T1-weighted contrast magnetic resonance imaging showing lesion hypointense on T1, heterogeneously hyperintense on T2 and dense contrast enhancing with edema and infiltrating pons. (e) Photomicrograph showing highly cellular glial tumor with high nuclear pleomorphism (H and E, ×40). (f) Tumor cell shows glial fibrillary acidic protein positive cytoplasmic processes (IP, ×40)
Case 3
A 30-year-old male patient presented with complaint of gait ataxia and visual blurring for 3 weeks. Examination revealed left cerebellar signs and a brun's nystagmus with coarse component to left. CT head demonstrated a hyperdense lesion in left anterior cerebellum spreading to middle cerebellar peduncle. MRI [
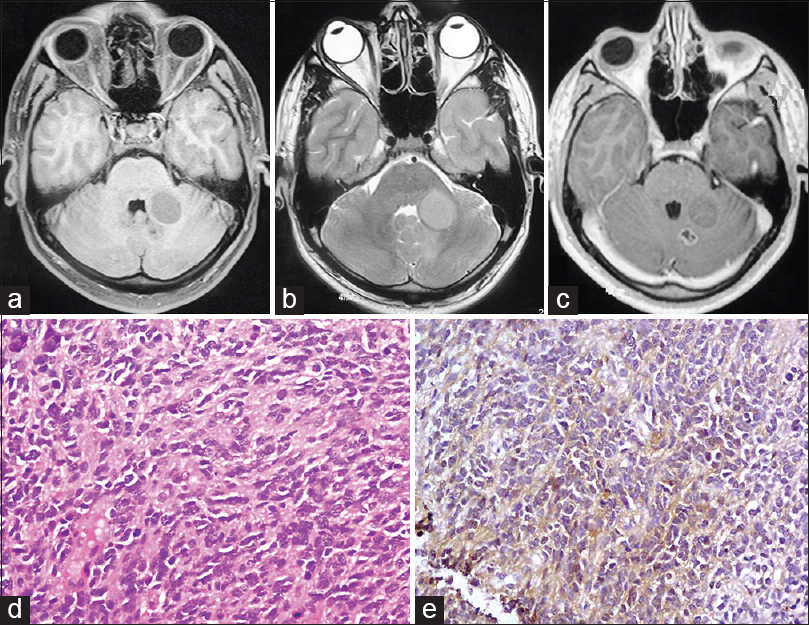
Figure 3
(a-c) T1-weighted, T2-weighted, and T1-weighted contrast axial images showing well-defined lesion at left middle cerebellar peduncle hypointense on T1, isointense on T2, predominantly nonenhancing on contrast with small posterior nodule showing ring enhancement and necrosis. (d) Photomicrograph showing cellular tumor composed of round, blue cells with high nuclear: Cytoplasmic ratio and high mitotic activity and rosette formation (H and E, ×40).(e) Tumor cells show faint cytoplasmic synaptophysin positivity (IP, ×40)
DISCUSSION
Glioblastomas in cerebellum account for <5% of all cerebellar astrocytomas.[
Tumor frequently extends beyond the enhancing part seen on imaging. Dissemination is believed to occur along ventricular wall and subarachnoid space[
The rapidity of onset of symptoms in these patients and previously reported patients harboring cerebellar high-grade lesions is alarming,[
It is possible that the natural behavior and, therefore, the mode of failure of therapy, of cerebellar high-grade lesions invading cerebellar peduncle differ from hemispheric lesions. The truly aggressive nature of these lesions and the dismal survival in the presence of limited overall reported cases so far raises question of appropriate line of management.[
Our experience with such high-grade lesions at cerebellar peduncle has made us believe that proper case selection is imperative as aggressive surgical approach may be hazardous. With limited OS and associated risk of working in vital brainstem regions, biopsy followed by chemoradiotherapy may be a better option in few selected patients.
CONCLUSION
Malignant cerebellar peduncle lesions present with short history, progress rapidly, and may have a dismal outcome despite surgery. The small number of overall reported cases in literature leaves much to be sorted out in coming years. Although the number of patients in our study is small, certainly the focus of this controversy is the poorly defined natural history of high-grade lesions at cerebellar peduncle. We share our experience of managing such patients and together with a thorough literature review, put forth a rarely described entity, the ideal management of which is controversial.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chung EJ, Jeun SS. Extra-axial medulloblastoma in the cerebellar hemisphere. J Korean Neurosurg Soc. 2014. 55: 362-4
2. Dohrmann GJ, Farwell JR, Flannery JT. Glioblastoma multiforme in children. J Neurosurg. 1976. 44: 442-8
3. Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006. 24: 1266-72
4. Endo H, Kumabe T, Kon H, Yoshimoto T, Nakasato Y. A case of primary cerebellar glioblastoma in childhood. No Shinkei Geka. 2002. 30: 1325-9
5. Jaiswal AK, Mahapatra AK, Sharma MC. Cerebellopointine angle medulloblastoma. J Clin Neurosci. 2004. 11: 42-5
6. Kopelson G, Linggood R. Cerebellar glioblastoma. Cancer. 1982. 50: 308-11
7. Kulkarni AV, Becker LE, Jay V, Armstrong DC, Drake JM. Primary cerebellar glioblastomas multiforme in children. Report of four cases. J Neurosurg. 1999. 90: 546-50
8. Lapras C, Patet JD, Lapras C, Mottolese C. Cerebellar astrocytomas in childhood. Childs Nerv Syst. 1986. 2: 55-9
9. Pang D, Ashmead JW. Extraneural metastasis of cerebellar glioblastoma multiforme. Neurosurgery. 1982. 10: 252-7
10. Reddy GD, Sen AN, Patel AJ, Bollo RJ, Jea A. Glioblastoma of the cerebellum in children: Report of five cases and review of the literature. Childs Nerv Syst. 2013. 29: 821-32
11. Salazar OM. Primary malignant cerebellar astrocytomas in children: A signal for postoperative craniospinal irradiation. Int J Radiat Oncol Biol Phys. 1981. 7: 1661-5
12. Shinoda J, Yamada H, Sakai N, Ando T, Hirata T, Hirayama H. Malignant cerebellar astrocytic tumours in children. Acta Neurochir (Wien). 1989. 98: 1-8
13. Steinberg GK, Shuer LM, Conley FK, Hanbery JW. Evolution and outcome in malignant astroglial neoplasms of the cerebellum. J Neurosurg. 1985. 62: 9-17