- Division of Neurosurgery, Department of Surgery, Faculty of Medicine, Jazan University, Jazan, Saudi Arabia,
- Department of Surgery, Division of Neurosurgery, University of West Indies and University Hospital of West Indies, Kingston, Jamaica, Caribbean,
- Department of Surgery, Division of Pediatric Neurosurgery, University of British Columbia and British Columbia Children’s Hospital, BC, Canada.
Correspondence Address:
Yahya H Khormi
Department of Surgery, Division of Pediatric Neurosurgery, University of British Columbia and British Columbia Children’s Hospital, BC, Canada.
DOI:10.25259/SNI_560_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yahya H Khormi, Ronette Goodluck Tyndall, Mandeep Tamber. Malignant clinical course of mycotic intracranial aneurysms in children: A review. 18-Apr-2020;11:71
How to cite this URL: Yahya H Khormi, Ronette Goodluck Tyndall, Mandeep Tamber. Malignant clinical course of mycotic intracranial aneurysms in children: A review. 18-Apr-2020;11:71. Available from: https://surgicalneurologyint.com/surgicalint-articles/9966/
Abstract
Background: Mycotic aneurysms are a rare in the pediatric population. The natural history of these lesions and their appropriate management strategies is controversial.
Case Description: A 13-year-old male presented with the sudden onset of a headache, vomiting, and fever. Inflammatory markers were elevated, and the blood culture was positive for Streptococcus viridans. When the computed tomography angiography (CTA) showed a ruptured mycotic aneurysm accompanied by multiple small unruptured aneurysms, he was started on antibiotics and underwent an urgent craniotomy. Despite negative blood cultures, the follow-up CTA showed further enlargement of the previously diagnosed aneurysms and a new right frontal aneurysm. The second and third craniotomies were, respectively, performed to resect the additional aneurysms. Pathologically, both aneurysmal walls were markedly inflamed and attenuated, suggesting the imminent risk of aneurysmal rupture. Following the total of three craniotomies, the patient had an uneventful postoperative course. Within 2 weeks, he regained baseline neurological function.
Conclusion: Mycotic aneurysms in children may follow a very malignant course. Aneurysms may grow, new ones may form, and repeated CTAs are required to direct further follow-up treatment.
Keywords: De novo aneurysm, Infectious aneurysm, Pseudoaneurysms
BACKGROUND
Brain mycotic aneurysms were first described in 1885 by William Osler.[
Patients usually present after an infarct or a hemorrhage. Common symptoms include fever, sepsis, headache, vomiting, deterioration of cardiac function, altered level of consciousness (e.g., due to mass effect from in hemorrhages), and neurological deficit attributed to hemorrhages/infarct.[
A high index of suspicion is required to establish the diagnosis of intracranial mycotic aneurysms in children. They most typically involve the distal circulation followed by the anterior cerebral artery distribution. The best test to diagnose a mycotic aneurysm is to perform a digital subtraction angiogram followed by computed tomography angiography (CTA). Other diagnostic studies that help confirm the diagnosis include positive blood culture, vegetations on an echocardiogram, and increased inflammatory markers (e.g., elevated white blood cell (WBC) count, higher C-reactive protein (CRP), and elevated erythrocyte sedimentation rate).[
Here, we present a 13-year-old male who developed multiple mycotic intracranial aneurysms requiring three successive craniotomies.
CASE PRESENTATION
A 13-year-old male, with a history of autism, mild developmental delay, and remote cardiac surgery, presented with the sudden onset of severe headaches and vomiting. He was febrile, but remained hemodynamically stable (e.g., normal Glasgow Coma Score) without a neurological deficit.
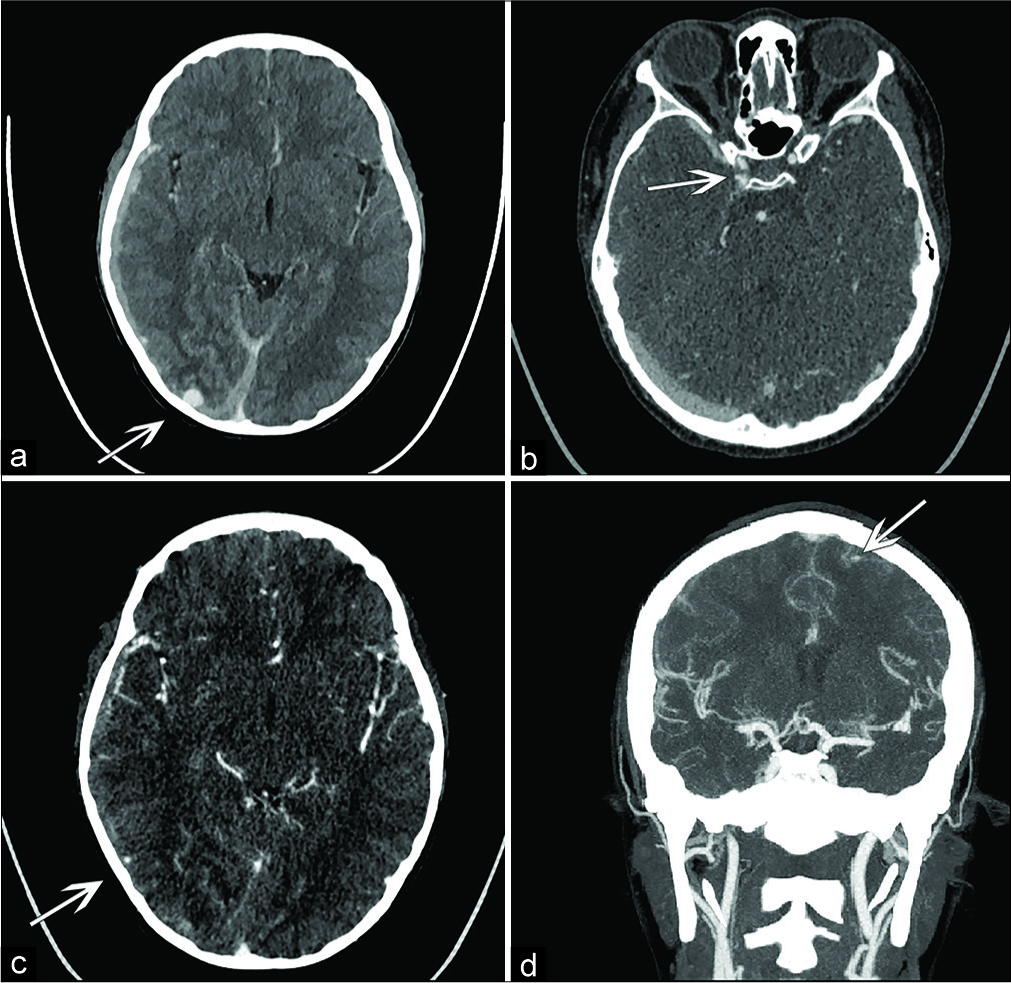
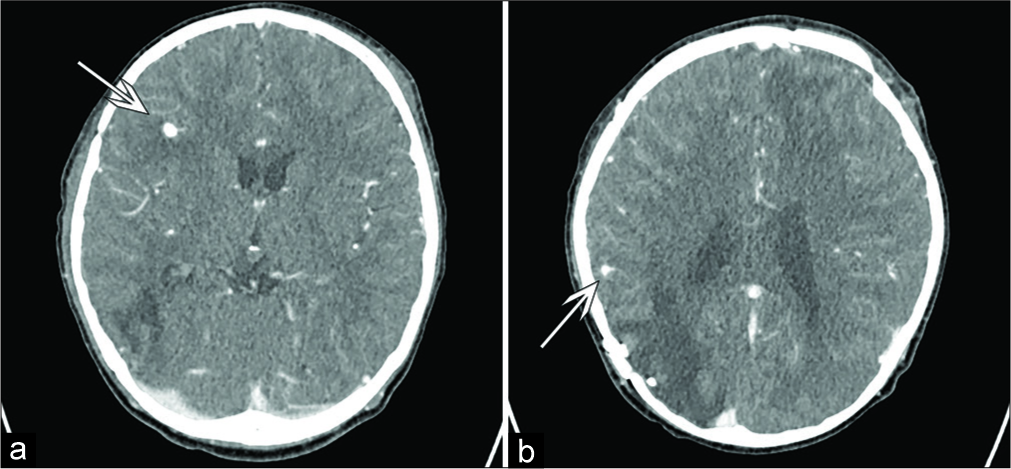
WBC and CRP levels were elevated, and the blood cultures were positive for S. viridans. The echocardiogram was normal; there were no vegetations. The brain computed tomography (CT) and CTA (angiogram) showed a right subdural hematoma and a focal right subarachnoid hemorrhage with a large mycotic aneurysm in the right occipital lobe without midline shift. There were also small mycotic aneurysms in the left frontal lobe, right parietal temporal junction, and right supraclinoid internal carotid artery (ICA) distributions [
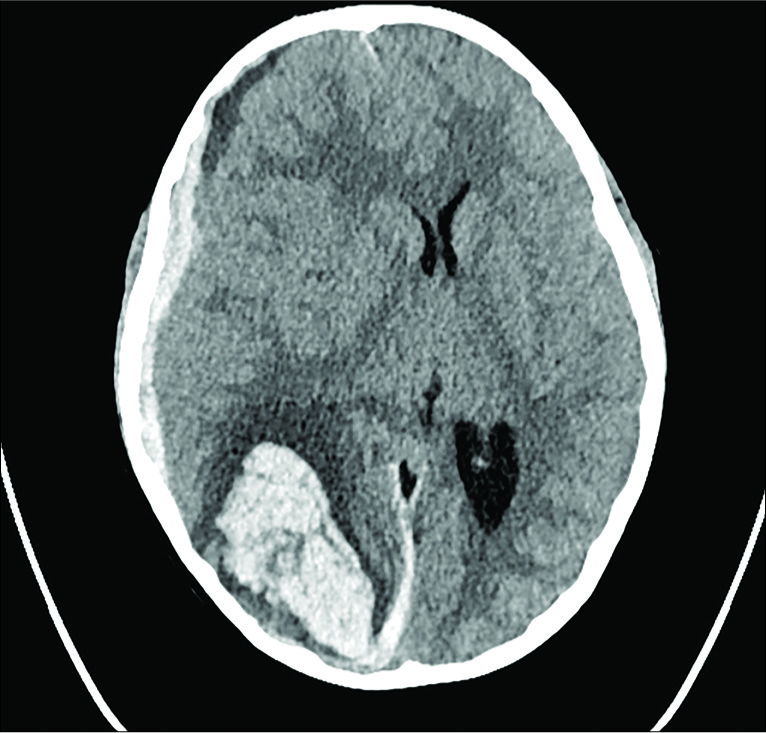
He was started empirically on intravenous (IV) ceftriaxone and vancomycin; this was later revised to ceftriaxone and gentamycin. The next morning, while awaiting right occipital aneurysm surgery, he woke up with a sudden recurrent headache. The right pupil became dilated and fixed; immediately, intubation with hyperventilation and hyperosmolar therapy was instituted. The brain CT showed a large right occipital, parietal, and posterior temporal lobe hemorrhage with uncal herniation [
After 48 h, the repeat CT/CTA showed no new hemorrhage or aneurysm formation; the largest aneurysm in the right occipital lobe was no longer evident, and there was no evidence of significant brain edema or midline shift. He was extubated on postoperative day 3 and recovered to baseline neurological function.
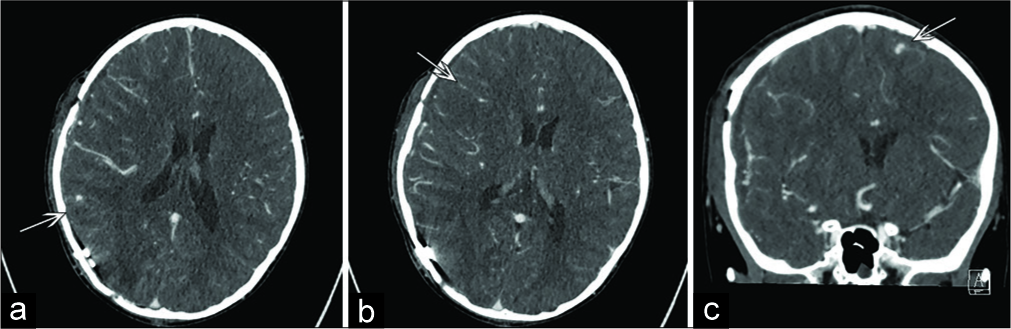
Two weeks later, however, the follow-up CTA demonstrated enlargement of the left frontal aneurysms and a new right frontal aneurysm [
One week later, the follow-up CTA demonstrated an increase in the size of the right deep frontal aneurysm (e.g., 2–4 mm), which was partly thrombosed, plus a small area of surrounding edema. The CTA one week after that confirmed further growth of the right frontal aneurysm to 6 mm, but no changes in the 4 mm aneurysm in the right parietal temporal junction or in the 2 mm focal bulge/nipple on the lateral wall of right supraclinoid ICA [
DISCUSSION
The natural history of mycotic aneurysms varies. Corr et al. reported that in 33% of cases, there is a progression of disease, 33% stabilize, and the remaining 33% decrease in size with antibiotics treatment. The history for saccular noninfectious aneurysms is markedly different, as they can rupture at any size.[
More recent reports favor early surgical management of mycotic aneurysms. Antibiotic therapy for the duration of 4–6 weeks or until blood cultures are negative is necessary regardless of whether endovascular versus surgical aneurysm treatment is instituted.[
Here, initial endovascular surgery was unfavorable due to the high risk of rupture during the early stages of mycotic aneurysm formation. However, in select cases, endovascular occlusion of a feeding parent artery sacrifice may be feasible if there is good collateral circulation since the risk of infarction.[
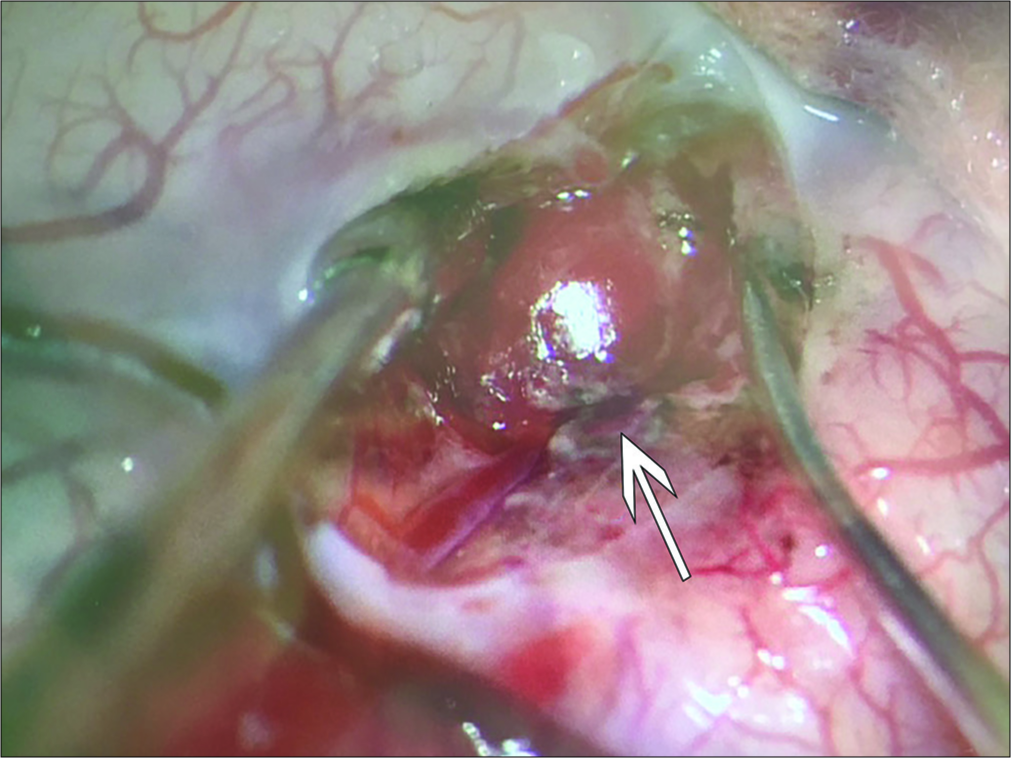
Microsurgery is with aneurysm resection/clot removal – decompression is presently utilized in 55% of patients with mycotic aneurysms. Alternatively, aneurysm clipping is suitable in aneurysms greater than 1 cm, for saccular aneurysms, and for those treated effectively with antibiotics for at least 2 weeks.[
Surgical adjuncts should include intraoperative image guidance and Doppler ultrasound to confirm the location of the mycotic aneurysms/focal hemorrhages.
CONCLUSION
Here, we reported how repeated CTAs documented multiple old and new mycotic aneurysms warranting three successive craniotomies on a 13-year-old male.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge Alexander Cheong for his contributions during the drafting of the manuscript.
References
1. Ducruet AF, Hickman ZL, Zacharia BE, Narula R, Grobelny BT, Gorski J. Intracranial infectious aneurysms: A comprehensive review. Neurosurg Rev. 2010. 33: 37-46
2. Flores BC, Patel AR, Braga BP, Weprin BE, Batjer HH. Management of infectious intracranial aneurysms in the pediatric population. Childs Nerv Sys. 2016. 32: 1205-17
3. Hamisch CA, Mpotsaris A, Timmer M, Reiner M, Stavrinou P, Brinker G. Interdisciplinary treatment of intracranial infectious aneurysms. Cerebrovasc Dis. 2016. 42: 493-505
4. Kannoth S, Thomas SV. Intracranial microbial aneurysm (infectious aneurysm): Current options for diagnosis and management. Neurocrit Care. 2009. 11: 120-9
5. Krings T, Geibprasert S, terBrugge KG. Pathomechanisms and treatment of pediatric aneurysms. Childs Nerv Syst. 2010. 26: 1309-18
6. Osler W. The gulstonian lectures on malignant endocarditis. Br Med J. 1885. 1: 577-9
7. Park W, Ahn JS, Park JC, Kwun BD, Lee DH. Treatment strategy based on experience of treating intracranial infectious aneurysms. World Neurosurg. 2017. 97: 351-9
8. Phuong LK, Link M, Wijdicks E. Management of intracranial infectious aneurysms: A series of 16 cases. Neurosurgery. 2002. 51: 1145-51