- Department of Neurosurgery, Henry Ford Hospital, Detroit, Michigan, USA
- Department of Neurology, Henry Ford Hospital, Detroit, Michigan, USA
Correspondence Address:
Richard Rammo
Department of Neurosurgery, Henry Ford Hospital, Detroit, Michigan, USA
DOI:10.4103/sni.sni_65_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Richard Rammo, Adam Robin, Jessin John, Aqueel Pabaney, Panayiotis Varelas, Max Kole. Management of acute subdural hematoma in a patient with portopulmonary hypertension on prostanoid therapy. 01-Aug-2017;8:161
How to cite this URL: Richard Rammo, Adam Robin, Jessin John, Aqueel Pabaney, Panayiotis Varelas, Max Kole. Management of acute subdural hematoma in a patient with portopulmonary hypertension on prostanoid therapy. 01-Aug-2017;8:161. Available from: http://surgicalneurologyint.com/surgicalint-articles/management-of-acute-subdural-hematoma-in-a-patient-with-portopulmonary-hypertension-on-prostanoid-therapy/
Abstract
Background:Treprostinil is a prostacyclin analog used to treat portopulmonary hypertension (PPHTN) and is one of several drugs shown to increase survival, but results in platelet dysfunction. Little is known about the management of patients on treprostinil who present with an acute subdural hematoma (aSDH). We describe such a case and offer our recommendations on management based on our experience and review of the literature.
Case Description:A 63-year-old, right-handed female with a history of PPHTN presented with severe headache and was found to have a large left aSDH with midline shift on imaging. She was admitted to the neurosurgical intensive care unit (ICU) where she developed hemiparesis and subsequently underwent emergent decompression. Postoperatively she improved, but several hours after became obtunded and imaging showed reaccumulation of the aSDH, which required reoperation. At 6 months postoperatively she had only a mild hemiparesis and was being reconsidered for treprostinil therapy as a bridge to liver transplant. Only one paper in the literature thus far has reported a patient with an aSDH managed with treprostinil. The authors achieved adequate intraoperative hemostasis without the use of platelet transfusion and lack of complications intraoperatively.
Conclusion:While concerns related to the risk of bleeding in surgery are valid, intraoperative hemostasis does not appear to be profoundly affected. Surgical intervention should not be delayed and prostanoid therapy discontinued, if possible, postoperatively. Patients should be placed in an intensive care setting with assistance from pulmonary specialists and close monitoring of neurological status and blood pressure.
Keywords: Acute subdural hematoma, portopulmonary hypertension, prostanoid therapy, pulmonary arterial hypertension, treprostinil
INTRODUCTION
The patient with acute subdural hematoma (aSDH) often constitutes a neurosurgical emergency; however, many cases are managed conservatively.[
CASE PRESENTATION
History
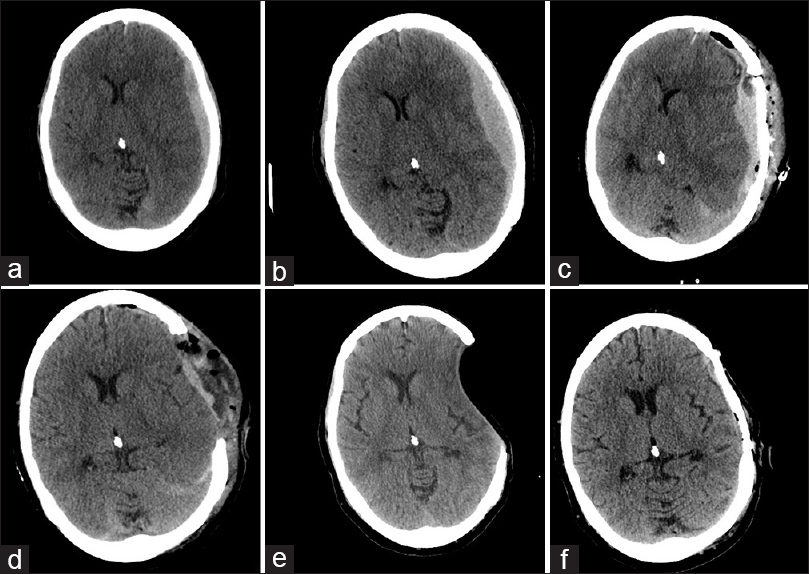
A 63-year-old, right-handed female presented with new complaints of shortness of breath, nausea, vomiting, and severe progressive headache over the course of 3 weeks. Her past medical history was complicated with a longstanding history of hepatitis C acquired from a blood transfusion in the 1970s. Unfortunately, she developed end-stage liver disease, cirrhosis, PPHTN, and coagulopathy. Her neurologic examination was grossly intact. A computed tomography (CT) scan of the head demonstrated a 13-mm, left-sided lentiform-shaped hyperdensity suggestive of subdural hematoma with midline shift of 7 mm and minimal transtentorial herniation [
Figure 1
Initial CT findings of left frontoparietal extra-axial hyperdensity and accompanying left-to-right shift of the ventricular system representing an acute SDH and its resultant mass effect (a) with subsequent progression (b). Following craniotomy and bone flap replacement there is recurrence (c). After reoperation for craniectomy, ventricles assume a more midline position (d). 2 months postoperatively, the brain has a sunken appearance and residual postoperative fluid has disappeared (e). Following cranioplasty the brain assumes its normal appearance (f)
Operative findings
She was taken to the operating room for a left frontotemporoparietal craniotomy and evacuation of the subdural clot. A small, bleeding pial artery that had coagulated was the likely source of the hematoma. Treprostinil infusion and sildenafil were continued throughout the surgery. Intraoperatively, hemostasis was obtained in routine fashion without undue burden from excessive bleeding. The brain was nicely pulsatile at the conclusion of surgery, a patch duraplasty loosely sewn, and the bone flap replaced. Tack-up sutures were used, and a subgaleal drain was left behind. Postoperatively she had an INR of 1.45 and a platelet count of 136,000/μL. She awoke immediately and began following commands. Her anisocoria and weakness improved. She became obtunded several hours after the operation and showed signs of herniation (Glasgow Coma Scale 4). A head CT scan demonstrated a large extra-axial hyperdense fluid collection with a midline shift of 19 mm in the same location as the SDH [
Postoperative course
A postoperative CT scan demonstrated resolution of midline shift and some persistent fluid within the operative bed [
DISCUSSION
The common pathophysiology in idiopathic pulmonary arterial hypertension (iPAH) and PPHTN results from diminished levels of endogenous eicosanoids produced in pulmonary vascular endothelial cells, which contributes to a high mean pulmonary arterial pressure and increased pulmonary vascular resistance.[
Prostacyclin analogs function primarily via the prostaglandin receptor on vascular smooth muscle cells.[
In the literature abrupt cessation of prostanoid therapy has been associated with rebound systemic and pulmonary hypertension.[
Even if rapid prostanoid cessation is not an option we recommend treating these patients aggressively — meaning limited unnecessary delay in reversal of coagulopathy prior to operative intervention. Safain et al. successfully treated a patient with iPAH on treprostinil with surgery and concluded that intraoperative hemostasis was not problematic, a finding we corroborate.
Specific steps can be taken during surgery to minimize the risk of reaccumulation requiring reoperation. The use of a subdural and subgaleal drain with consideration of craniectomy during the first operation may ameliorate the need to return to the OR if bleeding continues after initial surgery. We placed tack-up sutures in the first operation; however, the duraplasty was not closed at all the edges and a potential space remained between the bone and duraplasty that allowed blood to accumulate. It would have been safer to sew a watertight patch duraplasty with enough room for some expansion of the brain. With a duraplasty, aggressive tacking of the dura to the bone flap for obliteration of any potential epidural space would have made it more difficult for epidural hematoma formation. Placement of both subdural and subgaleal drains may have saved our patient from a repeat operation. During the second operation, we found the use of 2-0 Vicryl™ (Ethicon) on a CT-1 needle helped to ensure deep galeal, hemostatic stitches were placed in a swollen scalp, thus limiting the risk of epidural hematoma. The use of irrigation with a thrombin solution at the time of surgery for SDH has been described in patients with liver cirrhosis and is also an option as the technique resulted in an absolute risk reduction for recurrence of 20%.[
CONCLUSION
This report now represents the second published case of a surgically treated aSDH in a patient on prostanoid therapy and the first such case in end-stage liver disease and PPHTN. While concerns related to the risk of bleeding in surgery are valid, intraoperative hemostasis does not appear to be profoundly affected in patients taking prostanoid medications. Therefore, these patients should be operated on like any other patient with aSDH requiring surgery. If prostanoid therapy can be discontinued, we recommend careful monitoring in an ICU setting with assistance from neurocritical care and pulmonary specialists. There are a variety of critical care steps and technical nuances that can be employed to maximize patient care in this setting. The authors encourage other physicians to share their experiences with difficult cases so that patients may benefit.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Augoustides JG, Culp K, Smith S. Rebound pulmonary hypertension and cardiogenic shock after withdrawal of inhaled prostacyclin. Anesthesiology. 2004. 100: 1023-5
2. Bajsarowicz P, Prakash I, Lamoureux J, Saluja RS, Feyz M, Maleki M. Nonsurgical acute traumatic subdural hematoma: What is the risk?. J Neurosurg. 2015. 123: 1176-83
3. Barst RJ, Galie N, Naeije R, Simonneau G, Jeffs R, Arneson C. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J. 2006. 28: 1195-203
4. Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB. Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996. 334: 296-301
5. Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013. 62: D60-72
6. Gayat E, Mebazaa A. Pulmonary hypertension in critical care. Curr Opin Crit Care. 2011. 17: 439-48
7. LeVarge BL. Prostanoid therapies in the management of pulmonary arterial hypertension. Ther Clin Risk Manag. 2015. 11: 535-47
8. LeVarge BL, Channick RN. Inhaled treprostinil for the treatment of pulmonary arterial hypertension. Expert Rev Respir Med. 2012. 6: 255-65
9. Moncada S, Higgs EA, Vane JR. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977. 1: 18-20
10. .editorsNeurosurgery Knowledge Update: A Comprehensive Review. New York: Thieme; 2015. p.
11. Provencher S, Herve P, Jais X, Lebrec D, Humbert M, Simonneau G. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology. 2006. 130: 120-6
12. Rich S, Seidlitz M, Dodin E, Osimani D, Judd D, Genthner D. The short-term effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertension. Chest. 1998. 114: 787-92
13. Robbins IM, Cuiper LL, Stein CM, Wood AJ, He HB, Parker R. Angiotensin II mediates systemic rebound hypertension after cessation of prostacyclin infusion in sheep. J Appl Physiol (1985). 1998. 85: 731-7
14. Rubin LJPortopulmonary hypertension. UpToDate Post TW Ed UpToDate Walth. MA Available on: 2015 Dec 27. p.
15. Rubin LJ. Diagnosis and management of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004. 126: 7S-10S
16. Safain M, Shepard M, Rahal J, Kryzanski J, Hwang S, Roguski M. Successful management of an acute subdural hematoma in a patient dependent on continuous treprostinil infusion therapy. J Neurosurg. 2013. 118: 753-6
17. Safdar Z, Bartolome S, Sussman N. Portopulmonary hypertension: An update. Liver Transpl. 2012. 18: 881-91
18. Shimamura N, Ogasawara Y, Naraoka M, Ohnkuma H. Irrigation with thrombin solution reduces recurrence of chronic subdural hematoma in high-risk patients: Preliminary report. J Neurotrauma. 2009. 26: 1929-33
19. Skoro-Sajer N, Lang I. Treprostinil for the treatment of pulmonary hypertension. Expert Opin Pharmacother. 2008. 9: 1415-20
20. Torre-Amione G, Young JB, Durand J-B, Bozkurt B, Mann DL, Kobrin I. Hemodynamic effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients with class III to IV congestive heart failure. Circulation. 2001. 103: 973-80