- Professor of Clinical Neurosurgery, School of Medicine, State University of New York at Stony Brook, New York, and Chief of Neurosurgical Spine and Education, NYU Winthrop Hospital, NYU Winthrop NeuroScience/Neurosurgery, Mineola, New York 11501, United States.
DOI:10.25259/SNI_563_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. Many Intraoperative Monitoring Modalities Have Been Developed To Limit Injury During Extreme Lateral Interbody Fusion (XLIF/MIS XLIF): Does That Mean XLIF/MIS XLIF Are Unsafe?. 29-Nov-2019;10:233
How to cite this URL: Nancy E. Epstein. Many Intraoperative Monitoring Modalities Have Been Developed To Limit Injury During Extreme Lateral Interbody Fusion (XLIF/MIS XLIF): Does That Mean XLIF/MIS XLIF Are Unsafe?. 29-Nov-2019;10:233. Available from: http://surgicalneurologyint.com/surgicalint-articles/9775/
Abstract
Background: Extreme lateral interbody fusions (XLIF) and Minimally Invasive (MIS) XLIF pose significant risks of neural injury to the; lumbar plexus, ilioinguinal, iliohypogastric, genitofemoral, lateral femoral cutaneous, and subcostal nerves. To limit these injuries, many intraoperative neural monitoring (IONM) modalities have been proposed.
Methods: Multiple studies document various frequencies of neural injuries occurring during MIS XLIF/XLIF: plexus injuries (13.28%); sensory deficits (0-75%; permanent 62.5%); motor deficits (0.7-33.6%; most typically iliopsoas weakness (14.3%-31%)), and anterior thigh/groin pain (12.5-25%.-34%). To avoid/limit these injuries, multiple IONM techniques have been proposed. These include; using finger electrodes during operative dissection, employing motor evoked potentials (MEP), eliminating (no) muscle relaxants (NMR), and using “triggered” EMGs.
Results: In one study, finger electrodes for XLIF at L4-L5 level for degenerative spondylolisthesis reduced transient postoperative neurological symptoms from 7 [38%] of 18 cases (e.g. without IONM) to 5 [14%] of 36 cases (with IONM). Two series showed that motor evoked potential monitoring (MEP) for XLIF reduced postoperative motor deficits; they, therefore, recommended their routine use for XLIF. Another study demonstrated that eliminating muscle relaxants during XLIF markedly reduced postoperative neurological deficits/thigh pain by allowing for better continuous EMG monitoring (e.g. NMR no muscle relaxants). Finally, a “triggered” EMG study” reduced postoperative motor neuropraxia, largely by limiting retraction time.
Conclusion: Multiple studies have offered different IONM techniques to avert neurological injuries following MIS XLIF/XLIF. Does this mean that these procedures (e.g. XLIF/MIS XLIF) are unsafe?
Keywords: Extreme lateral interbody fusion (XLIF): Complications, Lumbar plexus injuries, Major injuries, Minor injuries, Nerve root injuries
INTRODUCTION
Extreme lateral interbody fusions (XLIF) and Minimally Invasive (MIS) XLIF place the lumbar plexus, ilioinguinal, iliohypogastric, genitofemoral, lateral femoral cutaneous, and subcostal nerves at risk of injury during surgery [
Nerves at Risk with XLIF
The lumbar plexus includes the L1-L4 nerves, and the subcostal nerve (T12). The sensory portion of the ilioinguinal nerve innervates the genital regions and some of the upper anterior/-medial thigh, while motor branches subserve the internal oblique and transversus abdominis muscles. The iliohypogastric nerve contributes to sensation over the lateral gluteal region, and provides motor innervation to the external/internal oblique, and transverse abdominus muscles. Sensation to the upper anterior thigh and genital regions is provided by the genitofemoral nerve, while sensation to the skin inferior to the iliac crest and gluteal regions is attributed to the lateral femoral cutaneous nerve. Lastly, the subcostal nerve (origin ventral ramus of T12 thoracic nerve) supplies motor innervation to the transversus abdominis, rectus abdominis, and pyramidalis.
Intraoperative Neural Monitoring to Avoid Neurological Deficits with XLIF/MIS XLIF
Use of Nerve Conduction Studies (NCS) and Electromyography (EMG)
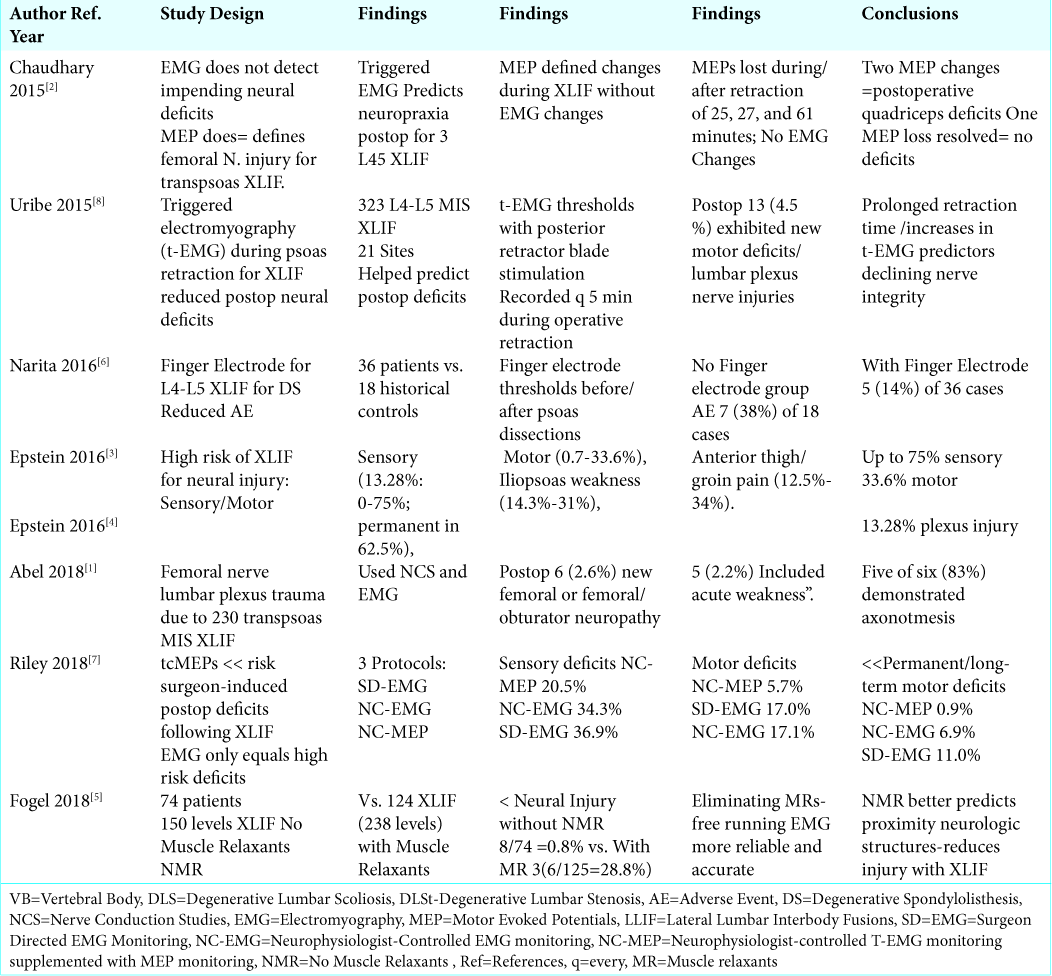
Abel et al. (2018) evaluated the extent of trauma to the femoral nerve and lumbar plexus occurring during 230 transpsoas MIS XLIF procedures utilizing different electrodiagnostic protocols [
Use of Finger Electrodes to Avoid Neurological Complications of XLIF
In 2016, Narita et al. studied whether using a finger electrode while performing L4-L5 XLIF for DS (degenerative spondylolisthesis) would reduce the incidence of new postoperative neurological deficits [
Motor Evoked Potential Monitoring (MEP) Decreases Deficits with XLIF
Several authors demonstrated that adding intraoperative MEP monitoring to EMG for XLIF, where EMG’s typically showed no changes, could reduce or limit the incidence of new postoperative neurological deficits [
No Muscle Relaxants (NMR) Avoids Neurological Injuries with XLIF
A typical complication of XLIF performed with muscle relaxants is proximal thigh pain and weakness involving the L3-L4, and L4-L5 levels. In 2018, Fogel et al. asked whether eliminating muscle relaxation during XLIF would reduce the risk of neural injury.[
Use of Triggered EMG to Predict Neuropraxia After MIS XLIF
Uribe et al. (2015) evaluated whether triggered EMG utilized during 323 L4-L5 MIS XLIF (from 21 study sites) during psoas retraction and XLIF would reduce postoperative neurological dysfunction [
CONCLUSION
Here we have presented multiple intraoperative neural monitoring (IONM) modalities developed to reduce nerve injuries following XLIF/MIS XLIF, including; finger electrodes, motor evoked potentials (MEP), no muscle relaxants (NMR), and using “triggered” EMGs [
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management.
References
1. Abel NA, Januszewski J, Vivas AC, Uribe JS. Femoral nerve and lumbar plexus injury after minimally invasive lateral retroperitoneal transpsoas approach: electrodiagnostic prognostic indicators and a roadmap to recovery. Neurosurg Rev. 2018. 41: 457-464
2. Chaudhary K, Speights K, McGuire K, White AP. Trans-cranial motor evoked potential detection of femoral nerve injury in trans-psoas lateral lumbar interbody fusion. J Clin Monit Comput. 2015. 29: 549-54
3. Epstein NE.editors. Extreme lateral lumbar interbody fusion: Do the cons outweigh the pros?. Surg Neurol Int. 2016. 7: S692-S700
4. Epstein NE.editors. High neurological complication rates for extreme lateral lumbar interbody fusion and related techniques: A review of safety concerns. Surg Neurol Int. 2016. 7: S652-S655
5. Fogel GR, Rosen L, Koltsov JCB, Cheng I. Neurologic adverse event avoidance in lateral lumbar interbody fusion: technical considerations using muscle relaxants. J Spine Surg. 2018. 4: 247-253
6. Narita W, Takatori R, Arai Y, Nagae M, Tonomura H, Hayashida T. Prevention of neurological complications using a neural monitoring system with a finger electrode in the extreme lateral interbody fusion approach. J Neurosurg Spine. 2016. 25: 456-463
7. Riley MR, Doan AT, Vogel RW, Aguirre AO, Pieri KS, Scheid EH. Use of motor evoked potentials during lateral lumbar interbody fusion reduces postoperative déficits. Spine J. 2018. 18: 1763-1778
8. Uribe JS, Isaacs RE, Youssef JA, Khajavi K, Balzer JR, Kanter AS.editors. Can triggered electromyography monitoring throughout retraction predict postoperative symptomatic neuropraxia after XLIF? Results from a prospective multicenter trial. Eur Spine J. 2015. 24: 378-85