- Department of Psychology, Faculty of Education and Psychology, Ferdowsi University of Mashhad, Mashhad, Iran
- Department of Neurosurgery, Epilepsy Center, Razavi Hospital, Mashhad, Iran,
- Department of Neurology, Epilepsy Center, Razavi Hospital, Mashhad, Iran.
Correspondence Address:
Bahram Ali Ghanbari Hashemabadi, Department of Psychology, Faculty of Education and Psychology, Ferdowsi University of Mashhad, Mashhad, Iran.
DOI:10.25259/SNI_49_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Seyyedeh Somayyeh Moshir Estekhareh1, Sajjad Saghebdoust2, Reza Zare2, Mohsen Aghaee Hakak3, Bahram Ali Ghanbari Hashemabadi1. Memory and executive functioning outcomes of selective amygdalohippocampectomy in patients with hippocampal sclerosis: A preliminary study in a developing country. 22-Apr-2022;13:161
How to cite this URL: Seyyedeh Somayyeh Moshir Estekhareh1, Sajjad Saghebdoust2, Reza Zare2, Mohsen Aghaee Hakak3, Bahram Ali Ghanbari Hashemabadi1. Memory and executive functioning outcomes of selective amygdalohippocampectomy in patients with hippocampal sclerosis: A preliminary study in a developing country. 22-Apr-2022;13:161. Available from: https://surgicalneurologyint.com/surgicalint-articles/11554/
Abstract
Background: Selective amygdalohippocampectomy (SA) is an effective treatment for drug-resistant cases of epilepsy due to hippocampal sclerosis (HS). However, its neurocognitive outcomes are inconsistent across the previous studies, pointing to potential location-specific confounders. Here, we investigated the neurocognitive outcomes of SA in an Iranian center recently adopting this approach.
Methods: Thirty adults (53.3% of females, age 31.4 ± 6.2 years) with drug-resistant epilepsy due to HS were included in the study. Patients were stratified into surgical (n = 15) and medical (n = 15) treatment groups based on their preferences. Neurocognitive function was assessed before and 6 months after intervention using Wisconsin Card Sorting Test (WCST), Wechsler Adult Intelligence Scale-Revised, and Wechsler Memory Scale- Third Edition (WMS-III). Postintervention performance changes were compared between the two groups, and predictors of worse postoperative outcomes were investigated.
Results: Longitudinal changes of performance in WMS-III and WCST were significantly different between the surgically and medically treated patients. Postoperative WMS-III performance showed an average 25% decline (mean ∆T2-T1 = –25.1%, T = –6.6, P P P
Conclusion: In our center, executive functioning improved or remained stable after SA, but memory functions declined moderately. The left-sided SA and higher education were associated with more severe decline in memory functions, highlighting the need for special considerations for these groups.
Keywords: Drug-resistant epilepsy, Executive function, Hippocampal sclerosis, Memory, Selective amygdalohippocampectomy
INTRODUCTION
Epilepsy is one of the most common disorders of the nervous system, with a lifetime prevalence of around 7.6 per 1000 individuals.[
In DRE cases, where the seizures originate from a focal and identifiable epileptogenic zone that is not crucial to important brain functions, surgical resection of this region can potentially lead to a cure.[
Temporal lobe epilepsy (TLE) most of the time presents resistance to medication, and just about 25% of the patients with MTLE respond to AED.[
With the development of surgical equipment and techniques in our country, we have recently started to perform SA in Razavi Hospital for the 1st time in East Iran. To the best of our knowledge, the neurocognitive outcomes of SA surgery have not yet been investigated in this country. Given the inconsistent findings of the previous studies and the potential location- specific confounders (e.g., the study population, surgical expertise, facilities, and postsurgical care) on surgical outcomes, here, we set to investigate the changes in cognitive and executive functioning in patients undergoing SA due to suspected HS in our center, as compared to medically treated controls.
MATERIALS AND METHODS
Participants
Thirty adults with DRE referred to Razavi Hospital, Mashhad, Iran, from 2014 to 2020 were recruited in this study. We included patients between the age of 20 and 40 years old who were proved to have DRE due to HS and were diagnosed by epilepsy MRI protocol and video EEG monitoring. The local ethics committee reviewed and approved the study protocol, and informed consent was obtained from each patient before their participation. The treatment was chosen based on patients’ preferences in discussion with their treating neurologist. Accordingly, 15 patients opted for SA surgery (cases), whereas 15 received medical treatment only (controls). The SA was performed through the Transsylvian approach to all the patients. We followed all the patients prospectively for 6 months to assess seizure outcome and neurocognitive function before the surgery (T1) and at the end of follow-up (T2).
Seizure outcome assessment
Seizure outcomes were assessed 6 months after SA surgery in all patients using the Engel classification.[
Neurocognitive assessment
Executive functioning, particularly the ability to reason, understand concepts, and react to a changing environment, was partly assessed using Wisconsin Card Sorting Test (WCST), the most commonly used test in this matter in our country.[
In addition, to assess performance in visual processing, verbal comprehension, and abstract reasoning, we used, another well-established test, the short form of Wechsler Adult Intelligence Scale-Revised (WAIS-R) with the following components: (1) “arithmetic,” which evaluates concentration, immediate memory, and basic mathematical skills, (2) “similarities,” which measures the ability of verbal conceptualization and abstract reasoning, (3) “picture completion,” which assesses visual concentration and visual processing performance, and (4) “block design,” which is a test of nonverbal intelligence, evaluating problem-solving, visual processing, and visual comprehension.[
We also used a validated Persian translation of the Wechsler Memory Scale-Third Edition (WMS-III) to evaluate memory performance.[
In all tests, raw scores were adjusted for age based on published normative data and were transformed to z-scores for ease of comparison.
Statistical analysis
In-house Python 3.7 scripts with pandas, NumPy, SciPy, and pingouin packages were used for the statistical analyses. Descriptive summaries of study variables were reported as mean ± standard deviation of continuous variables, or frequency and percent of categorical variables. The effect of SA on neurocognitive performances was assessed using mixed-design analysis of variance (ANOVA) with time as the within-subject factor and study group as the between- subjects factor. If group × time interactions were significant, post hoc paired t-tests were used to characterize the direction and magnitude of changes in each group. In addition, we investigated the association of changes in performance with the side of HS, level of education, gender, age, and preoperative performance for the neurocognitive measures with a significant postoperative change, using mixed- design ANOVAs and Pearson’s correlations limited to the SA group. It was ensured that the data met assumptions of parametric tests, and visual analysis was carried out as well. As there was no missing data, we did not use any method of missed data handling. The statistical significance threshold was set at P < 0.05.
RESULTS
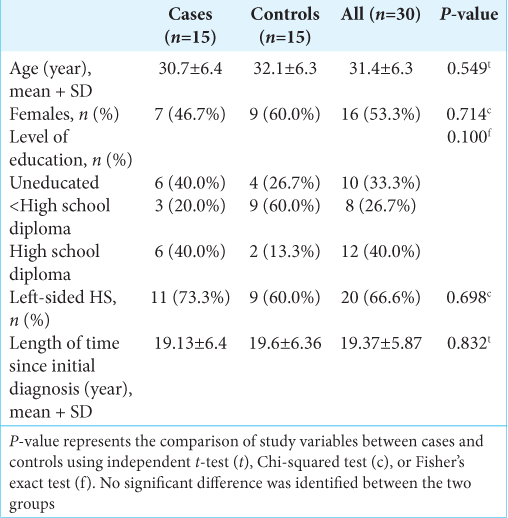
Thirty patients (53.3% of females, age 31.4 ± 6.2 years) with a suspected HS participated in the study. We observed no significant difference between the two SA and medically treated groups in their gender, age, level of education, and the more prominently affected hemisphere [
Seizure outcome
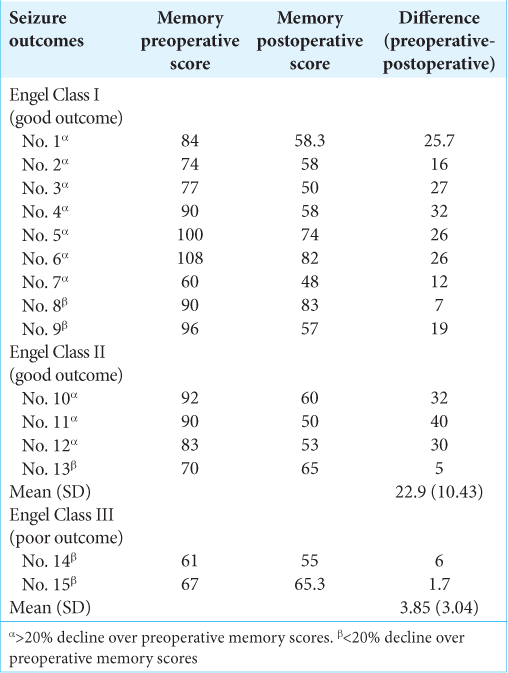
At the time of the neurocognitive assessment (6 months after surgery), 9 patients (60%) were seizure free and assigned to Engel Class I, and 7 patients (46.7%) were Engel Ia. Four patients (26.7%) were Class II and Class III was achieved in 2 patients (13.3%).
Memory outcome
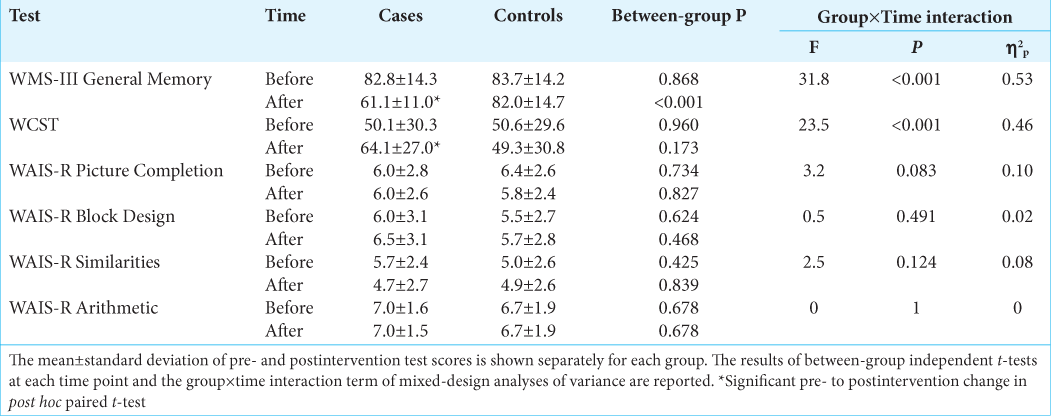
Baseline memory performances were not significantly different between cases and controls. However, postintervention changes of performance in memory function significantly differed between the two groups (F = 31.8, η2p = 0.53, and P < 0.001) [
Executive functioning outcome
There were no significant between-group differences in preintervention executive functioning performances. Postintervention changes of performance were significantly different between the two groups in WCST (F = 23.6, η2p = 0.46, and P < 0.001), but not in WAIS-R components [
DISCUSSION
In this prospective cohort, we investigated short-term seizure outcomes and neurocognitive functional outcomes of SA surgery for the 1st time in an Iranian center with a few years of experience in this type of surgery. Engel Class I (seizure freedom) was achieved in 9 patients (60%) at 6-month follow- up after transsylvian SA, whereas four and two patients were allocated to Engel II and Engel III, respectively. These results, apart from shorter follow-up duration, are approximately comparable to seizure freedom percentages in the previous studies following transsylvian SA of about 70%.[
Six months after SA, the patients showed an average 25% decline in memory performance, while the controls showed no significant change. In contrast, the functional outcome of SA with respect to executive functioning was generally good. As measured by WCST, the set-shifting ability was on average improved by 49% following SA but showed no significant change in medically treated patients. However, the longitudinal changes of other components of executive functioning, as measured by WAIS-R, including abstract reasoning, verbal comprehension, visual processing, and concentration, were not significantly different between the SA and control groups.
In line with our findings, several other studies have also reported a decrease in memory performance following selective or nonselective temporal lobe surgery in MTLE patients. In 2011, a meta-analysis of 12 studies reported an average rate of 44% and 20% decline in verbal memory performance in the left- and right-sided temporal lobe surgery, respectively.[
Contrary to the previous studies,[
The heterogeneity of post-SA cognitive outcomes can also be observed at the level of individuals, with some patients being more susceptible to memory deterioration after SA. For example, we found that a higher level of education was associated with more severe decline in memory function. Interestingly, this effect was attributable to a better baseline cognitive function of patients with higher education, and there was no difference in postoperative memory performances between educated and uneducated patients. The association of preoperative performance and education with verbal memory outcomes of temporal lesionectomy has also been shown in another cohort of patients with TLE and concurrent low-grade tumors.[
In addition, we showed that the left SA is associated with significantly more severe postoperative memory decline compared to the right SA. This left-sided asymmetry has also been observed in the previous studies on SA[
The improved postoperative executive functioning of our SA cases in set-shifting and their stability in other aspects of executive functioning is not unprecedented. Several other previous studies have also reported enhanced[
Our study had some limitations. As we have just recently developed the technique and expertise to perform SA in our center, this surgery is not routinely carried out, and only a few patients were available for this study. In addition, our study was not a randomized controlled trial, and patients received surgical or medical treatments based on their personal preferences. As a result, and because of our small sample size, we were limited in controlling for potential confounds, although we observed no significant differences in the characteristics and preoperative cognitive performances between the two groups. Furthermore, our cohort did not include patients with university/college-level education, and therefore, our results cannot be generalized to this group. Finally, it should be clearly stated that the 6-month follow- up is too short to draw any conclusion regarding the relationship between postoperative seizure outcomes and neurocognitive outcomes.
CONCLUSION
Taken together, in this prospective cohort on neurocognitive outcomes of SA surgery which was performed in an Iranian center recently adopting this approach, we showed that SA is generally safe or beneficial with regard to executive functioning but can lead to moderately impaired memory functions. The decline in memory functions was more severe in patients with higher education and those undergoing left-sided SA, highlighting the importance of additional considerations before selecting these patients for the surgery. In addition, a significant decline in memory scores seems to be associated with favorable seizure outcomes. Furthermore, this finding points to the need for identifying data-driven biological, imaging, or clinical predictors of post-SA neurocognitive outcomes.[
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Adada B. Selective amygdalohippocampectomy via the transsylvian approach. Neurosurg Focus. 2008. 25: E5
2. Beaton AE, Durnford A, Heffer-Rahn PE, Kirkham F, Griffin A, Gray WP. Transsylvian selective amygdalohippocampectomy in children with hippocampal sclerosis: Seizure, intellectual and memory outcome. Seizure. 2012. 21: 699-705
3. Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020. 54: 185-91
4. Boucher O, Dagenais E, Bouthillier A, Nguyen DK, Rouleau I. Different effects of anterior temporal lobectomy and selective amygdalohippocampectomy on verbal memory performance of patients with epilepsy. Epilepsy Behav. 2015. 52: 230-5
5. Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002. 35: 625-41
6. Carvajal F, Calahorra-Romillo A, Rubio S, Martín P. Verbal emotional memory laterality effect on amygdalohippocampectomy for refractory epilepsy. Brain Behav. 2020. 10: e01872
7. Cascino GD, Brinkmann BH. Advances in the surgical management of epilepsy: Drug-resistant focal epilepsy in the adult patient. Neurol Clin. 2021. 39: 181-96
8. Chelune GJ, Naugle RI, Lüders H, Sedlak J, Awad IA. Individual change after epilepsy surgery: Practice effects and base-rate information. Neuropsychology. 1993. 7: 41
9. Davies KG, Bell BD, Bush AJ, Wyler AR. Prediction of verbal memory loss in individuals after anterior temporal lobectomy. Epilepsia. 1998. 39: 820-8
10. Engel J.editors. Outcome with respect to epileptic seizures. Surgical Treatment of the Epilepsies. New York: Raven Press; 1993. p. 609-21
11. Foged MT, Vinter K, Stauning L, Kjær TW, Ozenne B, Beniczky S. Verbal learning and memory outcome in selective amygdalohippocampectomy versus temporal lobe resection in patients with hippocampal sclerosis. Epilepsy Behav. 2018. 79: 180-7
12. Giovagnoli AR, Casazza M, Ciceri E, Avanzini G, Broggi G. Preserved memory in temporal lobe epilepsy patients after surgery for low-grade tumour. A pilot study. Neurol Sci. 2007. 28: 251-8
13. Greenway MR, Lucas JA, Feyissa AM, Grewal S, Wharen RE, Tatum WO. Neuropsychological outcomes following stereotactic laser amygdalohippocampectomy. Epilepsy Behav. 2017. 75: 50-5
14. Hoyt AT, Smith KA. Selective amygdalohippocampectomy. Neurosurg Clin N Am. 2016. 27: 1-17
15. Ji GJ, Zhang Z, Zhang H, Wang J, Liu DQ, Zang YF. Disrupted causal connectivity in mesial temporal lobe epilepsy. PLoS One. 2013. 8: e63183
16. Kennepohl S, Sziklas V, Garver KE, Wagner DD, Jones-Gotman M. Memory and the medial temporal lobe: Hemispheric specialization reconsidered. Neuroimage. 2007. 36: 969-78
17. Kishima H, Kato A, Oshino S, Tani N, Maruo T, Khoo HM. Navigation-assisted trans-inferotemporal cortex selective amygdalohippocampectomy for mesial temporal lobe epilepsy; preserving the temporal stem. Neurol Res. 2017. 39: 223-30
18. Kongs SK, Thompson LL, Iverson GL, Heaton RK.editors. Wisconsin Card Sorting Test, 64 Card Version: WCST-64: PAR Lutz, FL. 2000. p.
19. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Hauser WA, Mathern G. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010. 51: 1069-77
20. Leonard G. Temporal lobe surgery for epilepsy: Neuropsychological variables related to surgical outcome. Can J Neurol Sci. 1991. 18: 593-7
21. Malikova H, Kramska L, Liscak R, Vojtech Z, Prochazka T, Mareckova I. Stereotactic radiofrequency amygdalo-hippocampectomy for the treatment of temporal lobe epilepsy: Do good neuropsychological and seizure outcomes correlate with hippocampal volume reduction?. Epilepsy Res. 2012. 102: 34-44
22. Mansouri A, Taslimi S, Abbasian A, Badhiwala JH, Akbar MA, Alotaibi NM. Surgical outcomes for medically intractable epilepsy in low and middle-income countries: A systematic review and meta-analysis. J Neurosurg. 2018. 131: 1-11
23. Martin RC, Sawrie SM, Edwards R, Roth DL, Faught E, Kuzniecky RI. Investigation of executive function change following anterior temporal lobectomy: Selective normalization of verbal fluency. Neuropsychology. 2000. 14: 501-8
24. Martin RC, Sawrie SM, Roth DL, Gilliam FG, Faught E, Morawetz RB. Individual memory change after anterior temporal lobectomy: A base rate analysis using regression based outcome methodology. Epilepsia. 1998. 39: 1075-82
25. Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annu Rev Psychol. 2006. 57: 27-53
26. Saed O, Rushan R, Moradi AR.editors. Investigating psychometric properties of wechsler memory scale-third edition for the students of tehran universities. Daneshvar Raftar. 2008. 15: 57-70
27. Saklofske DH, Schoenberg MR, Kreutzer JS, DeLuca J, Caplan B.editors. Wechsler adult intelligence scale (all versions). Encyclopedia of Clinical Neuropsychology. New York: Springer; 2011. p. 2675-80
28. Sander JW. Some aspects of prognosis in the epilepsies: A review. Epilepsia. 1993. 34: 1007-16
29. Schmeiser B, Wagner K, Schulze-Bonhage A, Elger CE, Steinhoff BJ, Wendling AS. Transsylvian selective amygdalohippocampectomy for mesiotemporal epilepsy: experience with 162 procedures. Neurosurgery. 2017. 80: 454-64
30. Sherman EMS, Wiebe S, Fay-McClymont TB, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L. Neuropsychological outcomes after epilepsy surgery: Systematic review and pooled estimates. Epilepsia. 2011. 52: 857-69
31. Spencer SS, Berg A, Vickrey B, Sperling M, Bazil C, Shinnar S. Initial outcomes in the multicenter study of epilepsy surgery. Neurology. 2003. 61: 1680-5
32. Stylianou P, Hoffmann C, Blat I, Harnof S. Neuroimaging for patient selection for medial temporal lobe epilepsy surgery: Part 1 Structural neuroimaging. J Clin Neurosci. 2016. 23: 14-22
33. Stylianou P, Kimchi G, Hoffmann C, Blat I, Harnof S. Neuroimaging for patient selection for medial temporal lobe epilepsy surgery: Part 2 functional neuroimaging. J Clin Neurosci. 2016. 23: 23-33
34. Tai XY, Bernhardt B, Thom M, Thompson P, Baxendale S, Koepp M. Neurodegenerative processes in temporal lobe epilepsy with hippocampal sclerosis: Clinical, pathological and neuroimaging evidence. Neuropathol Appl Neurobiol. 2018. 44: 70-90
35. Tanriverdi T, Dudley RW, Hasan A, Al Jishi A, Al Hinai Q, Poulin N. Memory outcome after temporal lobe epilepsy surgery: Corticoamygdalohippocampectomy versus selective amygdalohippocampectomy. J Neurosurg. 2010. 113: 1164-75
36. Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: A systematic review and meta-analysis. Brain. 2005. 128: 1188-98
37. Thom M. Hippocampal sclerosis in epilepsy: A neuropathology review. Neuropathol Appl Neurobiol. 2014. 40: 520-43
38. Thurman DJ, Logroscino G, Beghi E, Hauser WA, Hesdorffer DC, Newton CR. The burden of premature mortality of epilepsy in high-income countries: A systematic review from the mortality task force of the international league against epilepsy. Epilepsia. 2017. 58: 17-26
39. Tisser L, Palmini A, Paglioli E, Portuguez M, Azambuja N, da Costa JC. Pre and post-operative Wisconsin card sorting test performance in patients with temporal lobe epilepsy due to hippocampal sclerosis. Dement Neuropsychol. 2007. 1: 173-80
40. Vojtěch Z, Krámská L, Malíková H, Seltenreichová K, Procházka T, Kalina M. Cognitive outcome after stereotactic amygdalohippocampectomy. Seizure. 2012. 21: 327-33
41. Wachi M, Tomikawa M, Fukuda M, Kameyama S, Kasahara K, Sasagawa M. Neuropsychological changes after surgical treatment for temporal lobe epilepsy. Epilepsia. 2001. 42: 4-8
42. Wang C, Liu D, Yang Z, Yang Z. Clinical outcomes after medial temporal lobe epilepsy surgery: Anterior temporal lobectomy versus selective amygdalohippocampectomy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018. 43: 638-45
43. Wendling AS, Hirsch E, Wisniewski I, Davanture C, Ofer I, Zentner J. Selective amygdalohippocampectomy versus standard temporal lobectomy in patients with mesial temporal lobe epilepsy and unilateral hippocampal sclerosis. Epilepsy Res. 2013. 104: 94-104
44. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001. 345: 311-8
45. Yaşargil M, Wieser H, Valavanis A, Von Ammon K, Roth P. Surgery and results of selective amygdala-hippocampectomy in one hundred patients with nonlesional limbic epilepsy. Neurosurg Clin North Am. 1993. 4: 243-61
46. Yogarajah M, Mula M. Social cognition, psychiatric comorbidities, and quality of life in adults with epilepsy. Epilepsy Behav. 2019. 100: 106321
47. Yue J, Zhang CQ, Hou Z, Yang H. Subtemporal selective amygdalohippocampectomy in patients with mesial temporal lobe epilepsy: Systematic review of seizure and neuropsychological outcomes. Epilepsy Behav. 2020. 112: 107435