- Department of Neurosurgery Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran,
- Department of Pathology, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran,
- Department of Brain and Spinal Cord Injury Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran.
Correspondence Address:
Maysam Alimohamadi, Brain and Spinal Cord Injury Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran
DOI:10.25259/SNI_148_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohammad Hosseinzadeh1, Seyed Mehdi Ketabchi1, Seyed Ali Ahmadi2, Kasra Hendi1, Maysam Alimohamadi3. Meningioma as the host for metastatic breast cancer: A rare occurrence with important therapeutic impact. Surg Neurol Int 28-Jun-2021;12:314
How to cite this URL: Mohammad Hosseinzadeh1, Seyed Mehdi Ketabchi1, Seyed Ali Ahmadi2, Kasra Hendi1, Maysam Alimohamadi3. Meningioma as the host for metastatic breast cancer: A rare occurrence with important therapeutic impact. Surg Neurol Int 28-Jun-2021;12:314. Available from: https://surgicalneurologyint.com/surgicalint-articles/10922/
Abstract
Background: Tumor-to-tumor metastasis is a rare condition. There are few reports of metastatic tumors within intracranial tumors, including meningiomas. Since some metastatic tumors have osteoblastic imaging pattern, it is not always easy to differentiate them from meningioma on preoperative studies.
Case Description: A 60-year-old female referred to our center complaining about a progressive headache, nausea, and vomiting for the past month. She had a history of breast cancer treated with radical mastectomy (5 years ago) and adjuvant chemotherapy (until 1 year ago). Workups revealed a dural-based mass in the left temporobasal and midline subfrontal regions. Histopathological study showed breast cancer metastasis nests within the primary meningioma.
Conclusion: As the diagnosis of metastatic nests inside a benign tumor, drastically alters postoperative adjuvant treatments, a high index of suspicion is needed evaluating tumors from patients with a history of systemic neoplasms.
Keywords: Brain metastasis, Breast cancer, Intracranial metastasis, Meningioma, Skull base
INTRODUCTION
Tumor-to-tumor metastasis is a rare phenomenon. Since the first description by Fried in 1930,[
Metastases of systemic neoplasms to intracranial ones are even more rare, but have been reported to occur within different intracranial tumors including meningiomas.[
Hereby, we describe our experience with a rare case of metastasis within skull base meningioma in a patient already treated for ductal carcinoma of breast and review the pertinent literature.
CASE PRESENTATION
A 60-year-old female was referred to our center with a progressive headache started 1 month ago and nausea/ vomiting during the past week. Neurological examination was intact except for mild bilateral papilledema. She had a history of breast cancer (invasive ductal carcinoma, progesterone receptor [PR] positive, and HER-2 positive) treated by radical mastectomy 5 years ago, followed by adjuvant chemotherapy. She was regularly followed up by the oncologist. There was no evidence of metastatic spread and/or local recurrence and her cancer was reported to be in remission for 2 years ago.
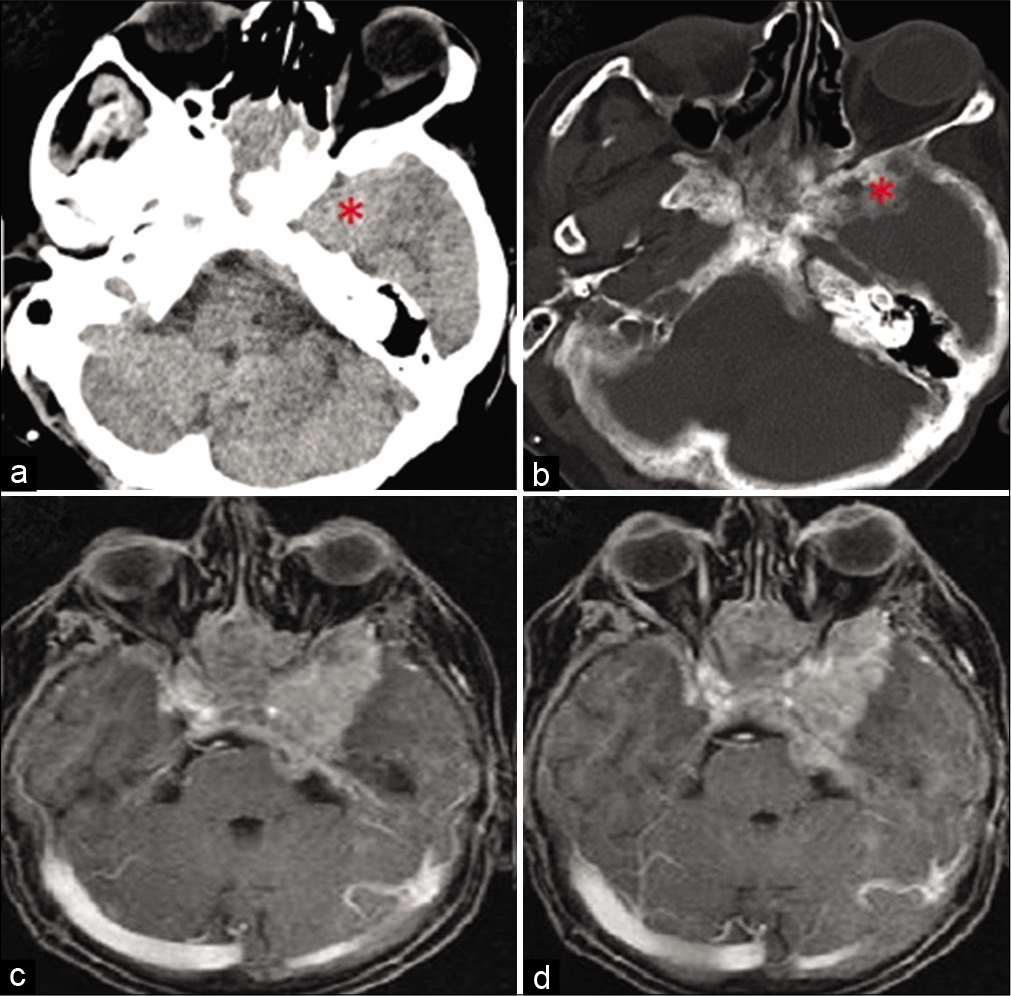
A brain CT was done [
Figure 1:
(a and b) Axial computed tomography scan showing a left temporobasal lesion with hypertrophic bone changes. (c and d) Axial postcontrast magnetic resonance imaging showing an extra-axial left temporobasal lesion with extension to the midline subfrontal region with poorly demarcated margins from the left temporal lobe.
After obtaining the patient’s informed consent, surgery was planned with the differential diagnoses of meningioma versus skull base metastasis. She was operated through a left frontopterional craniotomy. Tumor’s consistency was firm and the gross appearance was compatible with meningioma. The piecemeal removal of the tumor and microsurgical drilling of the involved bone were done as much as possible. The involved dura was also removed and reconstructed with pericranial fascia. After the surgery, the patient was admitted to neurosurgical ICU and after 24 h of close observation with no specific postsurgical complications, was transferred to the neurosurgery ward. The patient was discharged from the hospital 8 days after surgery without any neurological deficit.
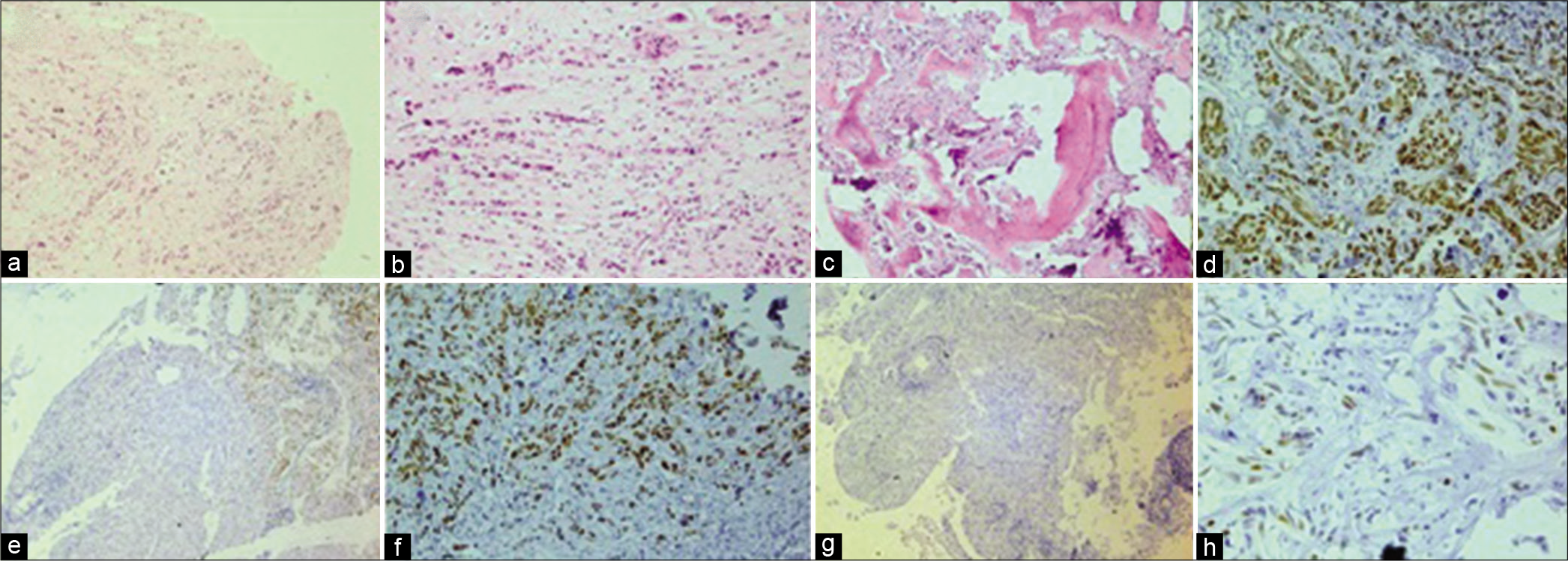
Histopathological study of the tumor revealed a WHO Grade 1 (WHO 2016 classification) meningioma containing nests of metastatic breast carcinoma in hematoxylin and eosin staining [
Figure 2:
(a) Hematoxylin and eosin staining (H & E, ×100) showing dura mater involved with meningioma and invaded by a tubule glandular forming tumor; an unusual feature for meningioma. (b) There are prominent nuclear atypia and “Indian filing” (H & E, ×200); both of them unfamiliar to meningioma and indicating invasive breast carcinoma. (c) Bone tissue infiltrated with atypical epithelial cells (H & E, ×100). (d) Immunohistochemistry staining (IHC, ×200) showing strong nuclear staining of tumor cells with GATA3 marker that is characteristic of breast carcinoma. (e) Negative staining of meningioma cells (left) compared to metastatic breast carcinoma (right) with GATA3 markers (IHC, ×100). (f) Strong positive staining of breast carcinoma cells with estrogen receptor (ER) marker (IHC, ×200). (g) Negative reaction of meningioma cells with ER marker (IHC, ×100). (h) Focal nuclear staining of both tumor cells with progesterone receptor marker (IHC, ×400).
Postoperative systemic metastasis workup (including PET scan) was clear and there were no signs of other metastatic foci. The patient was referred to oncologist for adjuvant chemoradiation therapy for skull base metastatic tumor.
DISCUSSION
The interrelation of meningioma and breast cancer is controversial in the existing literature. An early study by Schoenberg et al. reports an association between breast cancer and meningioma to such an extent that women with either condition have a higher risk of being diagnosed with the other one.[
Both meningioma and breast cancer are assumed as hormone-sensitive tumors frequently harboring PRs.[
Another possible reason for this correlation in incidence is suspected to be the usage of cranial imaging for staging and/ or follow-up, particularly among women with more advanced breast cancer.[
The first case of metastasis to meningioma was explained by Fried in 1930.[
Meningioma is the most common benign tumor hosting tumor-to-tumor metastases.[
Tumor-to-tumor metastasis could be mistaken with tumor collision. The phrase “collision tumors” depicts coexistence of two histologically different tumors neighboring in the same organ that invaded each other.[
Breast and lung cancers account for the source of 90% of brain and skull base metastasis. The same trend seems to exist for the sources of metastasis to intracranial tumors.[
In a review of the literature, Han et al. reported that systematic autopsy study of the cases with tumor-to-tumor metastasis usually shows a widely disseminated tumoral involvement in other organs. This is a very important clinical consideration for oncological management of patients with tumor-to-tumor metastasis, even those without an overt disseminated disease.[
CONCLUSION
Metastatic carcinoma, either alone or in combination with a primary intracranial tumor, should be suspected when facing intracranial masses among patients with a known history of systemic neoplasm. However, since there may be some overlapping imaging features between skull base meningiomas and some metastatic tumors, it is not always easy to differentiate between the two tumors preoperatively and a more detailed pathological study is needed for appropriate diagnosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bernstein RA, Grumet KA, Wetzel N. Metastasis of prostatic carcinoma to intracranial meningioma. Case report. J Neurosurg. 1983. 58: 774-7
2. Bernstein S. Uber karzinommetastase in einem Duraendotheliom. Zentralbl Allg Pathol. 1933. 58: 1633-66
3. Bucciero A, De Caro MD, Vizioli L, Carraturo S, Cerillo A, Tedeschi G. Metastasis of breast carcinoma to intracranial meningioma. Case report and review of the literature. J Neurosurg Sci. 1992. 36: 169-72
4. Campbell LV, Gilbert E, Chamberlain CR, Watne AL. Metastases of cancer to cancer. Cancer. 1968. 22: 635-43
5. Caroli E, Salvati M, Giangaspero F, Ferrante L, Santoro A. Intrameningioma metastasis as first clinical manifestation of occult primary breast carcinoma. Neurosurg Rev. 2006. 29: 49-54
6. Conzen M, Sollmann H, Schnabel R. Metastasis of lung carcinoma to intracranial meningioma. Case report and review of literature. Neurochirurgia (Stuttg). 1986. 29: 206-9
7. Criscitiello C, Disalvatore D, Santangelo M, Rotmensz N, Bazolli B, Maisonneuve P. No link between breast cancer and meningioma: Results from a large monoinstitutional retrospective analysis. Cancer Epidemiol Biomarkers Prev. 2014. 23: 215-7
8. Custer BS, Koepsell TD, Mueller BA. The association between breast carcinoma and meningioma in women. Cancer. 2002. 94: 1626-35
9. Dobbing J. Cancer to cancer. Guys Hosp Rep. 1958. 107: 60-5
10. Doron Y, Gruszkiewicz J. Metastasis of invasive carcinoma of the breast to an extradural meningioma of the cranial vault. Cancer. 1987. 60: 1081-4
11. Elmaci I, Ekinci G, Kurtkaya O, Sav A, Pamir MN.editors. Tumor in Tumor: Metastasis of Breast Carcinoma to Intracranial Meningioma. London, England: Sage; 2001. p.
12. Farrag A, Ansari J, Ali M, Sunbuli G, Kassem H, Al Hamad AA. Intracranial meningioma as primary presentation for an undiagnosed collision metastatic breast cancer: Case report and literature review. Mol Clin Oncol. 2018. 8: 661-4
13. Fried BM. Metastatic inoculation of a meningioma by cancer cells from a bronchiogenic carcinoma. Am J Pathol. 1930. 6: 47-52
14. Glass R, Hukku SR, Gershenhorn B, Alzate J, Tan B. Metastasis of lung adenosquamous carcinoma to meningioma: Case report with literature review. Int J Clin Exp Pathol. 2013. 6: 2625-30
15. Gyori E.editors. Metastatic Carcinoma in Meningioma. 1976. p.
16. Han HS, Kim EY, Han JY, Kim YB, Hwang TS, Chu YC. Metastatic renal cell carcinoma in a meningioma. J Korean Med Sci. 2000. 15: 593-7
17. Honma K, Hara K, Sawai T. Tumour-to-tumour metastasis. A report of two unusual autopsy cases. Virchows Arch A Pathol Anat Histopathol. 1989. 416: 153-7
18. Kim KH, Hong EK, Lee SH, Yoo H. Non small cell carcinoma metastasis to meningioma. J Korean Neurosurg Soc. 2013. 53: 43-5
19. Klotz S, Matula C, Pones M, Herac M, Grisold A, Hainfellner JA. Clinical neuropathology image 6-2018: Metastasis of breast carcinoma to meningioma. Clin Neuropathol. 2018. 37: 252-3
20. Lodrini S, Savoiardo M. Metastases of carcinoma to intracranial meningioma: Report of two cases and review of the literature. Cancer. 1981. 48: 2668-73
21. Milano MT, Grossman CE. Meningioma in breast cancer patients: Population-based analysis of clinicopathologic characteristics. Am J Clin Oncol. 2017. 40: 11-6
22. Moody P, Murtagh K, Piduru S, Brem S, Murtagh R, Rojiani AM. Tumor-to-tumor metastasis: Pathology and neuroimaging considerations. Int J Clin Exp Pathol. 2012. 5: 367-73
23. Ren Y, Cheng Y, Fan J, Zhang X, Yin S. A meningioma and breast carcinoma metastasis collision tumor. Br J Neurosurg. 2018. 4: 1-2
24. Schnegg JF, Gomez F, LeMarchand-Beraud T, de Tribolet N. Presence of sex steroid hormone receptors in meningioma tissue. Surg Neurol. 1981. 15: 415-8
25. Schoenberg BS, Christine BW, Whisnant JP. Nervous system neoplasms and primary malignancies of other sites: The unique association between meningiomas and breast cancer. Neurology. 1975. 25: 705-12
26. Smith TW, Schoene WC, Wang SY. Malignant carcinoid tumor metastatic to a meningioma. Cancer. 1981. 47: 1872-7
27. Sun LM, Lin CL, Sun S, Hsu CY, Shae Z, Kao CH. Long-term use of tamoxifen is associated with a decreased subsequent meningioma risk in patients with breast cancer: A nationwide population-based cohort study. Front Pharmacol. 2019. 10: 674
28. Turner N, Kaye AH, Paldor I. Metastases to meningioma-review and meta-analysis. Acta Neurochir (Wien). 2021. 163: 699-709