- Department of Neurosurgery, Coimbra University Hospital Centre, Portugal.
- Faculty of Medicine, University of Coimbra, Coimbra, Portugal.
Correspondence Address:
Daniela Matos
Department of Neurosurgery, Coimbra University Hospital Centre, Portugal.
Faculty of Medicine, University of Coimbra, Coimbra, Portugal.
DOI:10.25259/SNI_337_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daniela Matos1, Ricardo Pereira1,2. Meningioma-related subacute subdural hematoma: A case report. 29-Aug-2020;11:264
How to cite this URL: Daniela Matos1, Ricardo Pereira1,2. Meningioma-related subacute subdural hematoma: A case report. 29-Aug-2020;11:264. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10230
Abstract
Background: Meningiomas are the most frequent benign head tumors, although spontaneous hemorrhage is a rare form of presentation of such lesions. Of all possible bleeding locations associated with them, the subdural space is one of the most uncommon, with very few cases reported worldwide.
Case Description: A middle-aged woman presented with progressively worsening left-sided headache, initiated 2 weeks before, with no other complaints, denying any previous head trauma. Head computed tomography revealed a subacute left hemisphere subdural hematoma and left frontal, suggestive of meningioma on magnetic resonance imaging. Surgical treatment was performed with hematoma evacuation and lesion removal. Neuropathology showed a transitional meningioma with signs of hemorrhage. After surgery, no neurological deficits were registered, and headache abated.
Conclusion: As we could not identify any other cause for the subacute subdural hematoma, hemorrhage from the meningioma was the most probable cause, and thus, we decided to remove it along with clot evacuation. Based on neuropathological findings, we propose an alternative mechanism for this spontaneous hemorrhage from the meningioma, involving the place where the periphery of the lesion insertion, the dura mater as the origin of the hemorrhage. Knowledge of this association could help define the best treatment in such cases.
Keywords: Meningioma hemorrhage, Meningioma, Spontaneous bleeding, Subdural hematoma, Tumor hemorrhage
INTRODUCTION
The most frequent benign brain tumors, meningiomas, are generally slow-growing lesions that may have a broad clinical presentation depending on its location. Convexity meningiomas comprising 15% of these lesions, most frequently present with headache, followed by seizures, and other site-specific symptoms.[
CASE REPORT
A postmenopausal woman presented to our emergency room with an intense left-sided parieto- occipital pulsatile headache, with 2 weeks of evolution and progressive worsening in the previous 2 days and minimal response to acetaminophen. She had no other symptoms or history of head trauma. The neurological examination was normal.
The patient, hypocoagulated with acenocoumarol due to previous deep vein thrombosis, was also medicated with amlodipine for chronic hypertension.
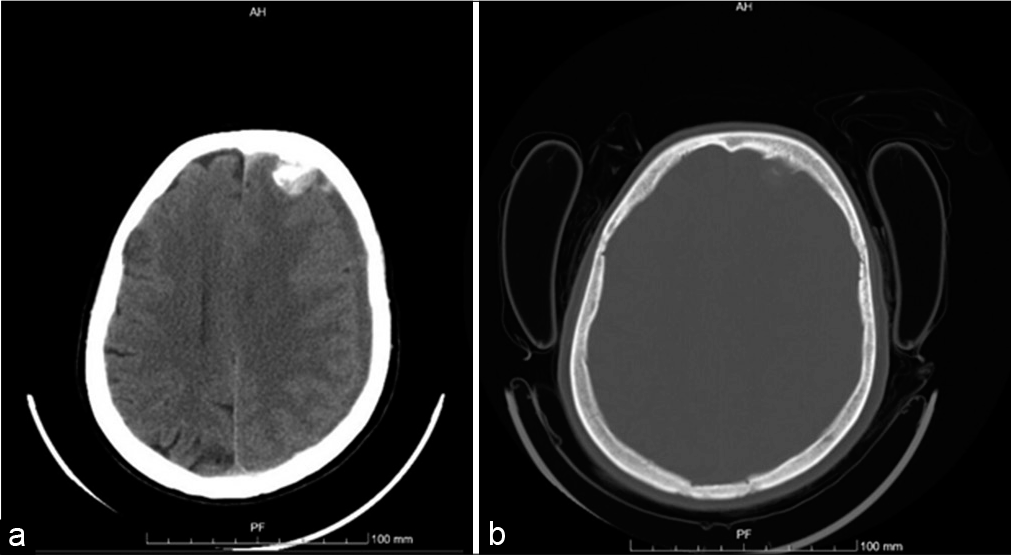
Head computed tomography (CT) showed a subacute left hemisphere and tentorial subdural hemorrhage with 17 mm of maximum width and signs of acute hemorrhage in the frontal region, as well as a left frontal dural-based lesion, well-demarcated, spontaneously hyperdense, and partially calcified, measuring 15 mm × 18 mm in diameter, causing surrounding edema and mass effect, inducing a 10 mm midline shift [
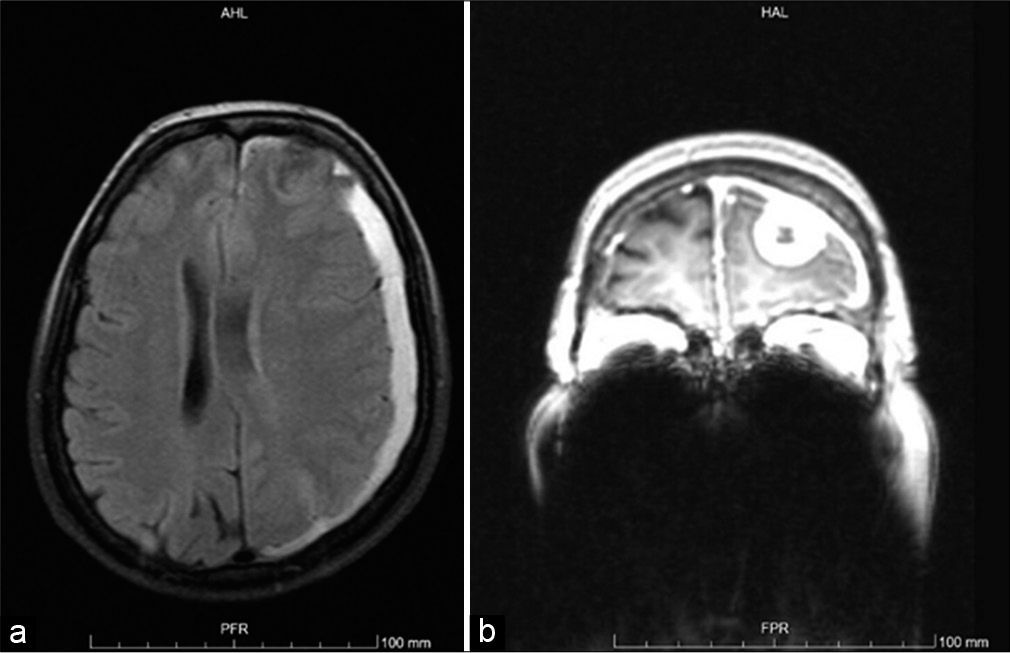
The patient was then admitted to the neurosurgery ward and head magnetic resonance imaging (MRI) confirmed an extra- axial left frontal lesion with adjacent dural enhancement after intravenous contrast [
Surgery with clot evacuation and lesion removal was the treatment option.
Operative procedure
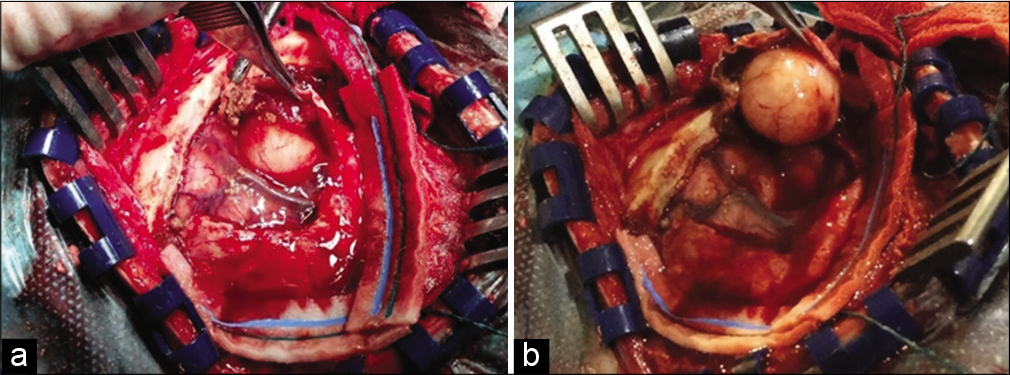
The patient underwent a small left frontal craniotomy using neuronavigational guidance. The dural opening revealed subacute subdural hemorrhage and a yellowish rounded lesion, very well delimited, that was carefully separated from [
Bony hyperostosis was drilled, and the bone flap regularized.
Histology
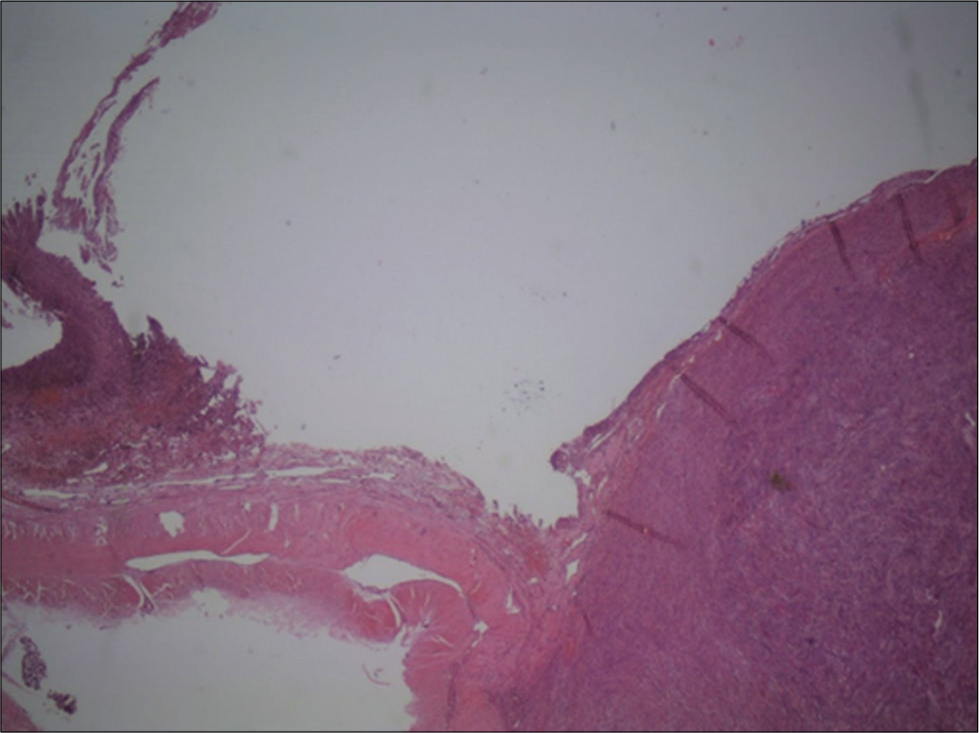
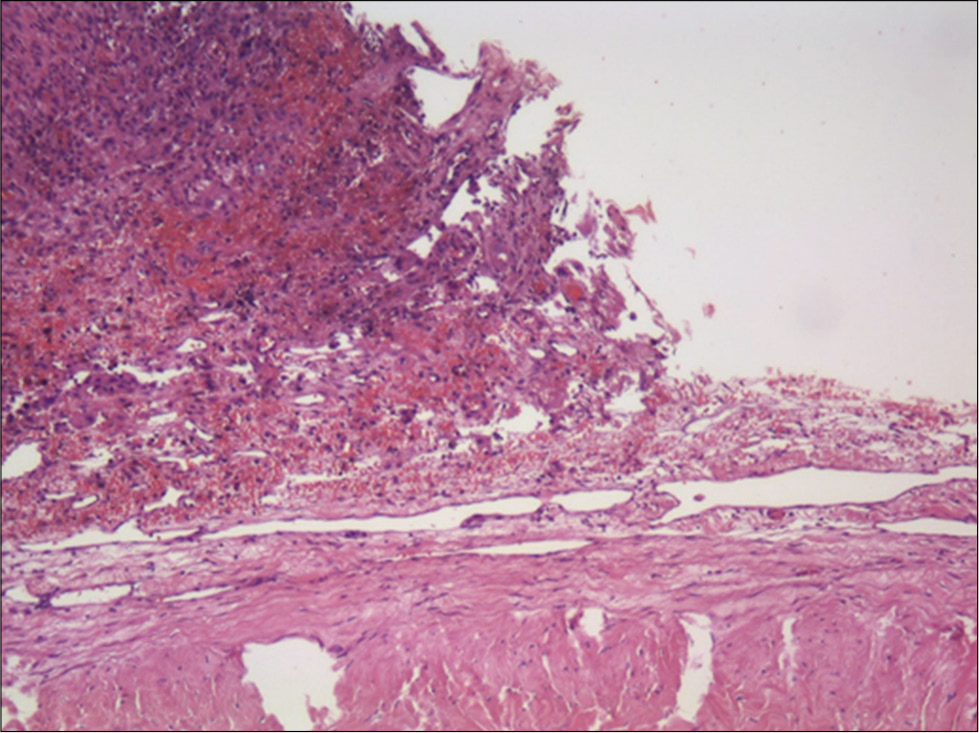
Histology showed a transitional meningioma (the World Health Organization Grade I/III) infiltrating the adjacent dura mater. Along with some areas of fibrosis and necrosis, a few areas of internal hemorrhage were identified. The adjacent dura mater showed signs of fibrovascular proliferation [
Postoperative outcome
The subdural drain was removed 24 h postsurgery. Steroids were slowly weaned postoperatively over the course of days, and seizure prophylaxis was continued for 1 month. Postoperative head CT showed a very good imaging result, with total subdural hematoma evacuation. After surgery, the patient noticed significant headache relief and needed no analgesics at discharge, maintaining a normal neurologic evaluation.
Postoperative head MRI showed complete lesion removal [
At the last follow-up appointment, 2 years after surgery, the patient remained asymptomatic.
DISCUSSION
The most common type of bleeding in meningiomas is subarachnoid hemorrhage, followed by intratumoral and intracerebral hemorrhage. Subdural hematoma, however, is extremely rare.[
We performed a thorough literature review and found that, in many cases, there was a lack of compelling data establishing a relationship between subdural collections and the presence of a convexity meningioma. This was particularly true in cases of chronic subdural collections in the elderly population and in cases where head trauma led to the diagnosis of both lesions. [
The underlying pathophysiologic mechanisms have been subject to several studies, although it remains unclear as to the reason why and which under the circumstances some meningiomas bleed.[
This patient was under amlodipine, which has a known vasodilator effect, reducing the peripheral resistance that added to the hypocoagulating effect of acenocoumarol which could possibly have influenced the development of the hemorrhage.
In this particular case, hypertension and hypocoagulation are considered risk factors causing the tumor to be more prone to bleeding when some minor tumor change occurs.[
Notwithstanding hypertension and hypocoagulatory states being common in the Portuguese population and thus present in many patients that harbor meningiomas, we found no other cases reported in our population, meaning those factors are probably not by themselves essential to this clinical presentation.
Considering neuropathological examination, the proximity of meningioma periphery and contiguous dura mater infiltrated by erythrocytes to the subdural hematoma membrane make this the probable origin of the hemorrhage. The rupture of a vessel in this transition zone could explain this phenomenon. Furthermore, the fact that the meningioma had minor intrinsic hemorrhages that seem to have no contact with the exterior layers of the lesion further supports this theory.
As we considered the bleeding of the meningioma as the probable cause for the acute subdural hemorrhage, we opted to remove both the hematoma and the lesion simultaneously in the same craniotomy.
CONCLUSION
Spontaneous subdural hemorrhage is an extremely rare presentation for convexity meningioma, but the possibility of an underlying lesion should always be entertained in cases of spontaneous intracranial hemorrhage, even those occurring in the subdural space.
Thorough characterization of possible causality between lesions is necessary, as it will greatly impact on therapeutic decisions.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agazzi S, Burkhardt K, Rilliet B. Acute haemorrhagic presentation of an intracranial meningioma. J Clin Neurosci. 1999. 6: 242-5
2. Aloraidi A, Abbas M, Fallatah B, Alkhaibary A, Ahmed M, Alassiri A. Meningioma presenting with spontaneous subdural hematoma: A report of two cases and literature review. World Neurosurg. 2019. 127: 150-4
3. Bloomgarden GM, Byrne TN, Spencer DD, Heafner MD. Meningioma associated with aneurysm and subarachnoid hemorrhage: Case report and review of the literature. Neurosurgery. 1987. 20: 24-6
4. Bosnjak R, Derham C, Popović M, Ravnik J. Spontaneous intracranial meningioma bleeding: Clinicopathological features and outcome. J Neurosurg. 2005. 103: 473-84
5. Chaskis C, Raftopoulos C, Noterman J, Flament-Durand J, Brotchi J. Meningioma associated with subdural haematoma: Report of two cases and review of the literature. Clin Neurol Neurosurg. 1992. 94: 269-74
6. Chen JW, Hoi-Sang U, Grafe MR. Unsuspected meningioma presenting as a subdural haematoma. J Neurol Neurosurg Psychiatry. 1992. 55: 167-8
7. Chonan M, Niizuma K, Koyama S, Kon H, Sannohe S, Kurotaki H. An operated case of a meningioma causing acute subdural hematoma. No Shinkei Geka. 2013. 41: 235-9
8. Hambra DV, Danilo de P, Alessandro R, Sara M, Juan GR. Meningioma associated with acute subdural hematoma: A review of the literature. Surg Neurol Int. 2014. 5: S469-71
9. Helle TL, Conley FK. Haemorrhage associated with meningioma: A case report and review of the literature. J Neurol Neurosurg Psychiatry. 1980. 43: 725-9
10. Jones NR, Blumbergs PC. Intracranial haemorrhage from meningiomas: A report of five cases. Br J Neurosurg. 1989. 3: 691-8
11. Kashimura H, Arai H, Ogasawara K, Beppu T, Kurose A, Ogawa A. Lipomatous meningioma with concomitant acute subdural hematoma. Neurol Med Chir. 2008. 48: 466-9
12. Kim JH, Gwak HS, Hong EK, Bang CW, Lee SH, Yoo H. A case of benign meningioma presented with subdural hemorrhage. Brain Tumor Res Treat. 2015. 3: 30-3
13. Kotwica Z, Zawirski M. Subdural hematoma caused by cerebral tumors. Zentralbl Neurochir. 1986. 47: 259-62
14. Koumtchev Y, Petkov S, Gozmanov G. Meningiom accompanied by a subdural hematoma. A case report. Review of 17 cases in the literature Folia Med (Plovdiv). 1993. 35: 61-3
15. Kouyialis AT, Stranjalis G, Analyti R, Boviatsis EJ, Korfias S, Sakas DE. Peritumoural haematoma and meningioma: A common tumour with an uncommon presentation. J Clin Neurosci. 2004. 11: 906-9
16. Lefranc F, Nagy N, Dewitte O, Baleriaux D, Brotchi J. Intracranial meningiomas revealed by non-traumatic subdural haematomas. A series of four cases. Acta Neurochir. 2001. 143: 977-83
17. Masoudi MS, Zafarshamspour S, Ghasemi-Rad M, Soleimani N, Rakhsha A, Lincoln C. Acute subdural hemorrhage of a convexity meningioma in the postpartum period; case report and literature review. Bull Emerg Trauma. 2019. 7: 324-9
18. Modesti LM, Binet EF, Collins GH. Meningiomas causing spontaneous intracranial hematomas. J Neurosurg. 1976. 45: 437-41
19. Niikawa S, Kawaguchi M, Sugimoto S, Hattori T, Ohkuma A. Meningioma associated with subdural hematoma case report. Neurol Med Chir (Tokyo). 1990. 30: 169-72
20. Pressman E, Penn D, Patel N. Intracranial hemorrhage from meningioma: 2 Novel risk factors. World Neurosurg. 2020. 135: 217-21
21. Ravindran K, Gaillard F, Lasocki A. Spontaneous subdural haemorrhage due to meningioma in the post-partum setting. J Clin Neurosci. 2017. 39: 77-9
22. Sanai N, Sughrue ME, Shangari G, Chung K, Berger MS, McDermott MW. Risk profile associated with convexity meningioma resection in the modern neurosurgical era. J Neurosurg. 2010. 112: 913-9
23. Sato K, Sugawara T, Fujiwara S, Mizoi K, Yoshimoto T. A case of small meningioma with acute subdural hematoma. No Shinkei Geka. 1989. 17: 687-90
24. Shimizu J, Tazawa T, Park-Matsumoto YC, Katano T, Akiba Y, Kuroiwa T. Meningioma associated with acute subdural hematoma: A case report. No Shinkei Geka. 1998. 26: 743-7
25. Spektor S, Ashkenazi E, Israel Z. Intracranial haemorrhage from a meningioma in a patient receiving aspirin prophylaxis: A case report. Acta Neurochir. 1995. 134: 51-3
26. Tokunaga T, Kuboyama M, Kojo N, Matsuo H, Shigemori M, Kuramoto S. A case of acute subdural hematoma associated with convexity meningioma. No Shinkei Geka. 1988. 16: 1389-93
27. Ueno M, Nakai E, Naka Y, Kido T, Itakura T, Komai N. Acute subdural hematoma associated with vacuolated meningioma case report. Neurol Med Chir. 1993. 33: 36-9
28. Wang HC, Wang BD, Chen MS, Li SW, Chen H, Xu W. An underlying pathological mechanism of meningiomas with intratumoral hemorrhage: Undifferentiated microvessels. World Neurosurg. 2016. 94: 319-27
29. Wu A, Garcia MA, Magill ST, Chen W, Vasudevan HN, Perry A. Presenting symptoms and prognostic factors for symptomatic outcomes following resection of meningioma. World Neurosurg. 2018. 111: e149-59