- Department of Neurosurgery Tufts Medical Center, Boston, Massachusetts, United States.
- Department of Pathology and Laboratory Medicine, Tufts Medical Center, Boston, Massachusetts, United States.
Correspondence Address:
Mina G. Safain, Department of Neurosurgery, Tufts Medical Center, Boston, Massachusetts, United States.
DOI:10.25259/SNI_1139_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Harleen Saini1, Andy Y. Wang1, Jacob J. Kosarchuk1, Furkan Yigitbilek2, Laleh Montaser Kouhsari2, Knarik Arkun2, Ron I. Riesenburger1, Mina G. Safain1. Metal allergy hypersensitivity after posterior thoracic spinal fusion: A case report and review of the literature. 30-Dec-2021;12:635
How to cite this URL: Harleen Saini1, Andy Y. Wang1, Jacob J. Kosarchuk1, Furkan Yigitbilek2, Laleh Montaser Kouhsari2, Knarik Arkun2, Ron I. Riesenburger1, Mina G. Safain1. Metal allergy hypersensitivity after posterior thoracic spinal fusion: A case report and review of the literature. 30-Dec-2021;12:635. Available from: https://surgicalneurologyint.com/surgicalint-articles/11306/
Abstract
Background: Spine surgeons rarely consider metal allergies when placing hardware, as implants are thought to be inert.
Case Description: A 32-year-old male presented with a skin rash attributed to the trace metal in his spinal fusion instrumentation. Patch testing revealed sensitivities to cobalt, manganese, and chromium. He underwent hardware removal and replacement with constructs of commercially pure titanium. His skin findings resolved at 2 weeks after surgery and were stable at 6 weeks.
Conclusion: Hypersensitivity to metal (i.e., metal allergy) should be considered before performing instrumented spinal fusions.
Keywords: Hypersensitivity, Instrumentation, Metal allergy, Spinal fusion, Thoracic
BACKGROUND
Instrumentation used during spinal fusions is traditionally thought to be inert, and thus, spine surgeons rarely consider metal hypersensitivities. Metal allergies have been described in other surgeries such as total hip and knee arthroplasties, with an allergy to nickel reported as the most common, followed by palladium, cobalt, potassium dichromate, and vanadium.[
CASE PRESENTATION
A 32-year-old male presented with a rash 2 years after a thoracic spinal fusion for a T5-T6 fracture-dislocation with complete spinal cord injury (T5 sensory level, ASIA A). He had undergone an uncomplicated T4-T8 posterior pedicle screw and rod fusion. The instrumentation consisted mainly of titanium with small quantities of other metals (i.e., including aluminum, vanadium, and cobalt chrome) [
Removal and replacement of instrumentation
As the patient had not formed a complete arthrodesis at T5-T6 and had the presence of metal allergies, he had the prior instrumentation removed and replaced with commercially pure titanium [
Pathology
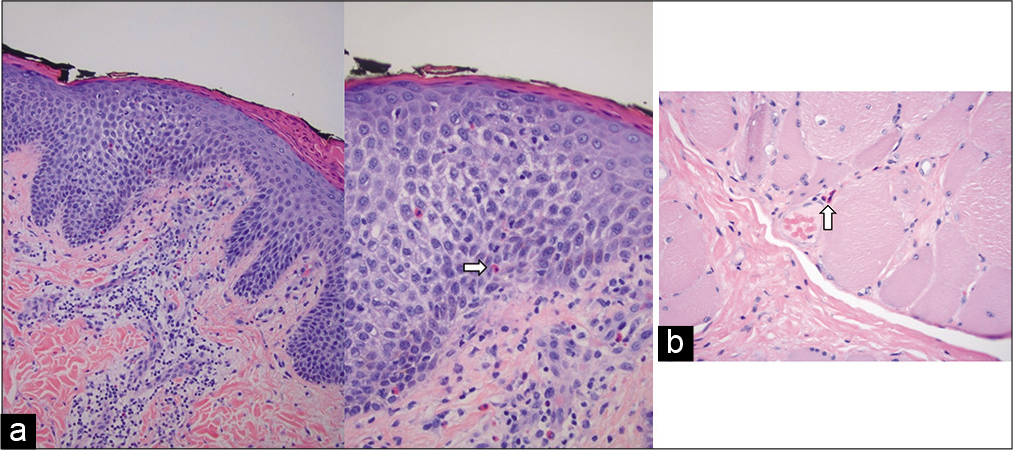
Pathological findings compatible with an allergic eczematous dermatitis on hematoxylin and eosin staining of skin plaques demonstrated spongiotic dermatitis with multifocal parakeratosis scale crust and superficial to middermal perivascular lymphocytic infiltrate with occasional eosinophils [
Figure 3:
Histopathological examination. (a) Hematoxylin and eosin-stained sections from skin plaques demonstrated spongiotic dermatitis with multifocal parakeratosis scale crust and superficial to mid-dermal perivascular lymphocytic infiltrate with occasional eosinophils (arrow). These findings are compatible with an allergic eczematous dermatitis. Left: ×20, right: ×40. (b) Muscle sections demonstrated chronic inflammation, occasional eosinophils (arrow), basophilic fibers, atrophy, nuclear clumping, and increased internal nuclei. Hematoxylin and eosin stain at ×40.
DISCUSSION
Pedicle screw and rod constructs are often placed without consideration of metal hypersensitivity. These sensitivities are often attributed to trace metals that result in a delayed-type IV immune reaction, although a type III reaction may also play a role.[
Screening for metal allergies before instrumented spinal fusions
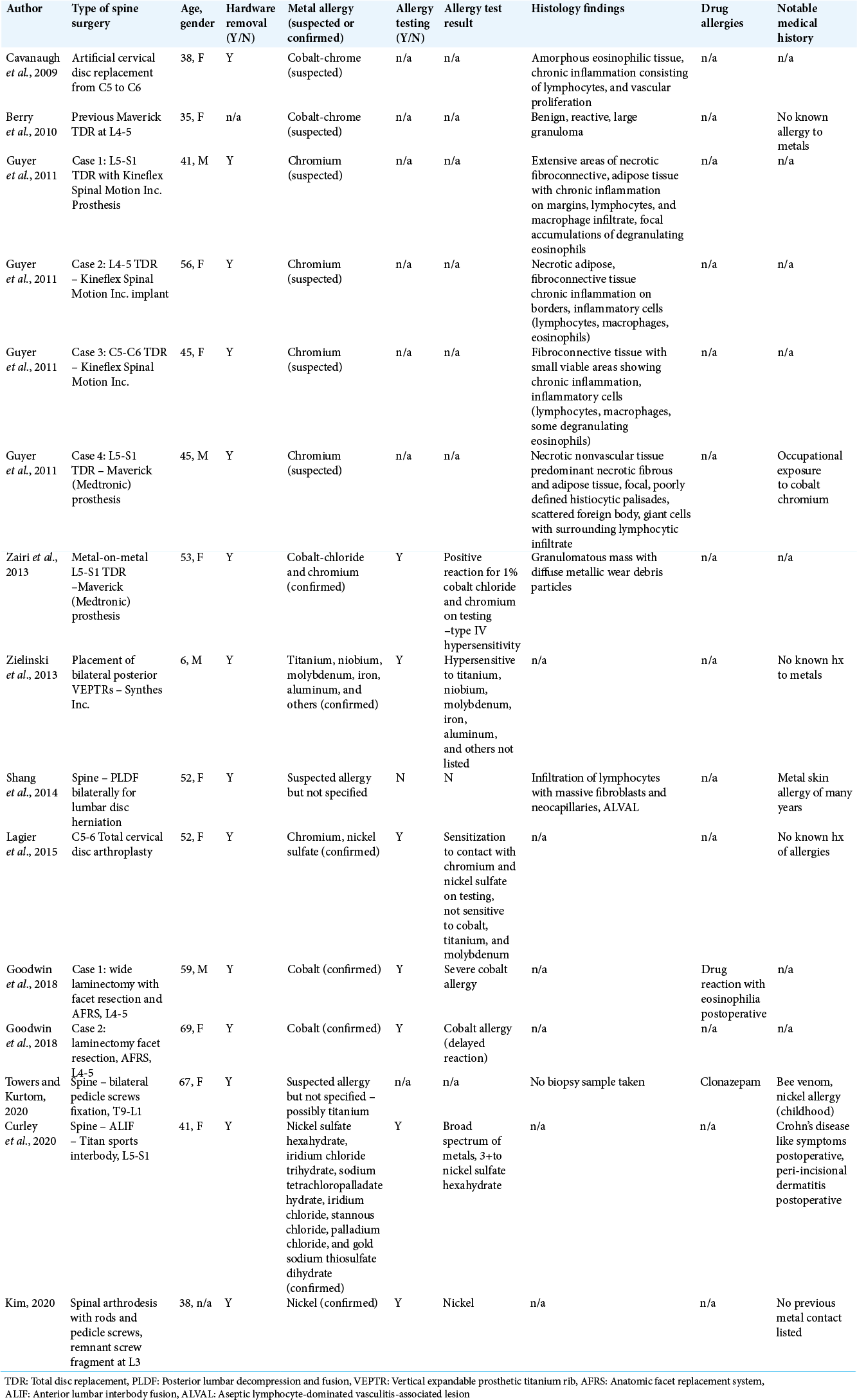
Spine surgeons should consider the risk of metal hypersensitivity before implanting spinal instrumentation. In elective cases, the patient’s medical history should be scrutinized for past metal hypersensitivity or occupational exposure to metals. Of the 15 case reports of allergy to spinal implants, the majority (87%) were due to disc arthroplasty (most commonly containing cobalt and chromium), with only two cases of pedicle screw instrumentation [
Testing for metal allergy
Patients with hypersensitivity reactions may be difficult to differentiate from the much more common wound infection complications. Where allergy to an implant is considered, patch testing should be performed. If hypersensitivity to the implant is confirmed, the implant should be removed and replaced with other available systems such as commercially pure titanium, hydroxyapatite, stainless steel, calcium phosphate, polymethylmethacrylate bone cement, carbon fiber-reinforced polyetheretherketone, and tantalum.[
CONCLUSION
Before instrumented fusions, patients should be screened for a history of metal allergies, and allergy patch tested if necessary. For those with symptoms/signs of a metal allergy to spinal instrumentation, removal of the construct is a key, with or without replacement if a pseudoarthrosis is present.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank Walter C. Dent for helping to obtain the photos used in the figures.
References
1. Berry MR, Peterson BG, Alander DH. A granulomatous mass surrounding a Maverick total disc replacement causing iliac vein occlusion and spinal stenosis: A case report. J Bone Joint Surg Am. 2010. 92: 1242-5
2. Cavanaugh DA, Nunley PD, Kerr EJ, Werner DJ, Jawahar A. Delayed hyper-reactivity to metal ions after cervical disc arthroplasty: A case report and literature review. Spine (Phila Pa 1976). 2009. 34: E262-5
3. Curley KL, Krishna C, Maiti TK, McClendon J, Bendok BR. Metal hypersensitivity after spinal instrumentation: When to suspect and how to treat. World Neurosurg. 2020. 139: 471-7
4. Fage SW, Muris J, Jakobsen SS, Thyssen JP. Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermatitis. 2016. 74: 323-45
5. Goodwin ML, Spiker WR, Brodke DS, Lawrence BD. Failure of facet replacement system with metal-on-metal bearing surface and subsequent discovery of cobalt allergy: Report of 2 cases. J Neurosurg Spine. 2018. 29: 81-4
6. Guyer RD, Shellock J, MacLennan B, Hanscom D, Knight RQ, McCombe P. Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction: Diagnosis and treatment experience in four cases. Spine (Phila Pa 1976). 2011. 36: E492-7
7. Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009. 67: 182-8
8. Kim J. A rare case of delayed hypersensitivity reaction to metal ions secondary to a remnant pedicle screw fragment after spinal arthrodesis. Acta Orthop Traumatol Turc. 2020. 54: 461-4
9. Kręcisz B, Kieć-Świerczyńska M, Chomiczewska-Skóra D. Allergy to orthopedic metal implants-a prospective study. Int J Occup Med Environ Health. 2012. 25: 463-9
10. Lagier M, Briere M, Giorgi H, Fuentes S, Blondel B, Tropiano P. Delayed hypersensitivity reaction after cervical disc replacement: A case report. Orthop Traumatol Surg Res. 2015. 101: 643-5
11. Shang X, Wang L, Kou D, Jia X, Yang X, Zhang M. Metal hypersensitivity in patient with posterior lumbar spine fusion: A case report and its literature review. BMC Musculoskelet Disord. 2014. 15: 314
12. Towers WS, Kurtom K. Rare systemic response to titanium spinal fusion implant: Case report. Cureus. 2020. 12: e7109
13. Warburton A, Girdler SJ, Mikhail CM, Ahn A, Cho SK. Biomaterials in spinal implants: A review. Neurospine. 2020. 17: 101-10
14. Zairi F, Remacle JM, Allaoui M, Assaker R. Delayed hypersensitivity reaction caused by metal-on-metal total disc replacement. J Neurosurg Spine. 2013. 19: 389-91
15. Zielinski J, Lacy TA, Phillips JH. Carbon coated implants as a new solution for metal allergy in early-onset scoliosis: A case report and review of the literature. Spine Deform. 2014. 2: 76-80