- Department of Neurosurgery, National Hospital Organization Himeji Medical Center, Himeji, Hyogo, Japan
Correspondence Address:
Kohei Morita
Department of Neurosurgery, National Hospital Organization Himeji Medical Center, Himeji, Hyogo, Japan
DOI:10.4103/2152-7806.185784
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Morita K, Oda M, Koyanagi M, Saiki M. Metastatic brain tumor from urothelial carcinoma of the prostatic urethra. Surg Neurol Int 07-Jul-2016;7:
How to cite this URL: Morita K, Oda M, Koyanagi M, Saiki M. Metastatic brain tumor from urothelial carcinoma of the prostatic urethra. Surg Neurol Int 07-Jul-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/metastatic-brain-tumor-urothelial-carcinoma-prostatic-urethra/
Abstract
Background:Urothelial carcinoma occurs in the bladder, upper urinary tract, and lower urinary tract, including prostatic urethra. A majority of the reported cases of intracranial metastasis from urothelial carcinoma originates from the bladder and upper urinary tract. Brain metastasis from urothelial carcinoma of the prostatic urethra has not yet been reported in the literature.
Case Description:A 72-year-old male presented with a metastatic brain tumor and a 3-year history of urothelial carcinoma of the prostatic urethra treated with cystourethrectomy and chemotherapy with gemcitabine-cisplatin. Pathological diagnosis for tumor removal was compatible with metastatic brain tumor from urothelial carcinoma.
Conclusion:Brain metastasis from urothelial carcinoma of the prostatic urethra has not yet been reported in the literature. It is an extremely rare case, however, we should be careful of brain metastasis during follow-up for urothelial carcinoma in the lower urinary tract.
Keywords: Metastatic brain tumor, prostatic urethra, urothelial carcinoma
INTRODUCTION
Brain metastasis is the most common form of brain tumor in adults, mainly occurring from the lung, breast, and colon. Brain metastasis derived from the urinary bladder and urinary tract cancer is rare.[
CASE REPORT
A 72-year-old, right-handed man with a past medical history of urothelial carcinoma of the prostatic urethra presented with left homonymous hemianopsia and headaches. The patient was previously diagnosed with urothelial carcinoma of the prostatic urethra when he was 68 years old. At that time, he was treated with total cystourethrectomy. The pathological diagnosis revealed urothelial carcinoma with no metastasis to the lymph node but invasion into the vein [
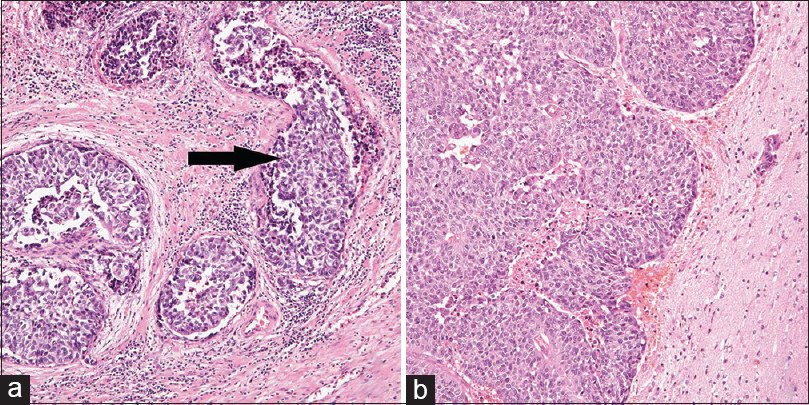
Figure 1
(a) A 68-year-old patient was treated with total cystourethrectomy. Hematoxylin-eosin staining of the prostatic urethra reveals urothelial carcinoma. The arrow indicates groups of urothelial carcinoma cells invading into the vein. (b) Hematoxylin-eosin staining of metastatic brain tumor shows groups of well-differentiated urothelial carcinoma cells with necrosis, which is the same pathological findings of the origin of the tumor, the prostatic urethra. The tumor was attached to the dura mater
A follow-up abdominal CT scan 1 year after cystourethrectomy showed metastasis of urothelial carcinoma to the lateral external iliac of the right lymph node. He was treated with six cycles of gemcitabine and cisplatin chemotherapy. During post-chemotherapy follow-up, other metastases were not found.
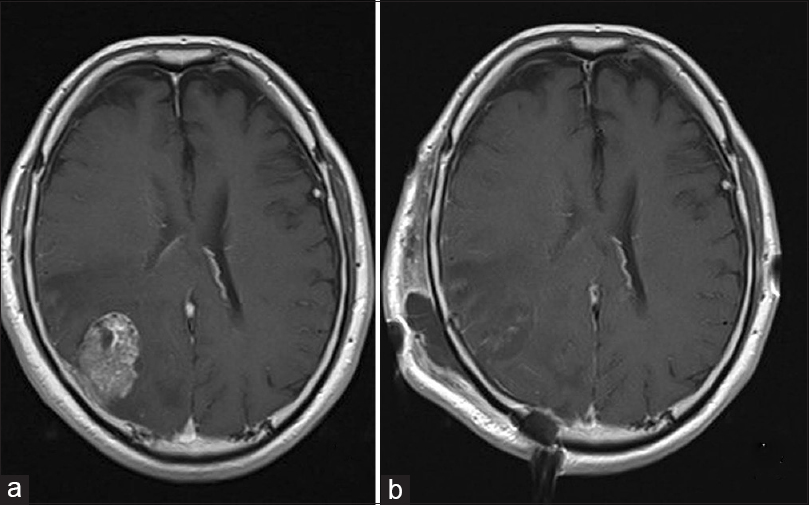
Three years after cystourethrectomy, the patient presented with left homonymous hemianopsia and headaches. Neurological examination upon admission revealed no other neurological deficits. Magnetic resonance imaging (MRI) showed an irregularly-shaped, heterogeneously-enhanced mass with gadolinium enhancement on T1-weighted images [
Postoperative MRI showed gross total removal of the tumor [
DISCUSSION
We reported here a rare case of a metastatic brain tumor from urothelial carcinoma of the lower urinary tract with venous invasion and metastasis to the lymph nodes. Urothelial carcinoma is a common type of cancer in western countries[
Although urothelial carcinoma from the lower urinary tract has the same pathological features as urothelial carcinoma from the upper urinary tract, there are some differences in the mechanism of metastasis.[
For the treatment of urothelial carcinoma, the primary form of chemotherapy is cisplatin-based schemes such as gemcitabine-cisplatin (GC); methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC); or paclitaxel, cisplatin, and gemcitabine (PCG).[
Similar to other metastatic brain tumors, the treatment for cerebral metastasis of urothelial carcinoma may follow the general guidelines for metastatic brain tumors. For a single large-sized lesion (>3 cm), particularly with a mass effect (>1 cm midline shift), surgical resection should be considered with/without whole brain radiotherapy (WBRT). WBRT has been accepted as one of the treatments for metastatic brain tumors.[
Cases of metastatic urothelial carcinoma usually show poor outcomes,[
Although metastatic brain tumors of urothelial carcinoma of the prostatic urethra are rare, accounting for this metastatic mechanism, we should consider the possibility of brain metastasis. In addition, a reported case of a long-term survivor showed that good control of metastasis resulted in a good prognosis.[
Considering this case, taking a frequent follow-up and treatment with chemotherapy, including the GC protocol, is recommended after surgical treatment.
CONCLUSION
This is a case of a metastatic brain tumor of urothelial carcinoma of the prostatic urethra and lower urinary tract. It could be treated with surgical resection, chemotherapy, and radiation therapy as with other metastatic brain tumors of urothelial carcinoma. Considering a poor prognosis of the tumor, we should be careful to consider brain metastasis during the follow-up of patients with urothelial carcinoma in the lower urinary tract as with other urothelial carcinomas.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. A component of this case was presented at the spring conference of Japan Neurosurgery Association in Kinki legion, Osaka, April 14, 2015.
References
1. Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg. 1940. 112: 138-49
2. Castellano D, Puente J, de Velasco G, Chirivella I, Lopez-Criado P, Mohedano N. Safety and effectiveness of vinflunine in patients with metastatic transitional cell carcinoma of the urothelial tract after failure of one platinum-based systemic therapy in clinical practice. BMC Cancer. 2014. 14: 779-
3. Chaichana KL, Rao K, Gadkaree S, Dangelmajer S, Bettegowda C, Rigamonti D. Factors associated with survival and recurrence for patients undergoing surgery of cerebellar metastases. Neurol Res. 2014. 36: 13-25
4. Clatterbuck RE, Sampath P, Olivi A. Transitional cell carcinoma presenting as a solitary brain lesion: A case report and review of the world literature. J Neurooncol. 1998. 39: 91-4
5. Fervenza FC, Wolanskyj AP, Eklund HE, Richardson RL. Brain metastasis: An unusual complication from prostatic adenocarcinoma. Mayo Clin Proc. 2000. 75: 79-82
6. Moon KS, Jung S, Lee KH, Hwang EC, Kim IY. Intracranial metastasis from primary transitional cell carcinoma of female urethra: Case report & review of the literature. BMC Cancer. 2011. 11: 23-
7. Palmieri D, Chambers AF, Felding-Habermann B, Huang S, Steeg PS. The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007. 13: 1656-62
8. Rades D, Meyners T, Veninga T, Stalpers LJA, Schild SE. Hypofractionated whole-brain radiotherapy for multiple brain metastases from transitional cell carcinoma of the bladder. Int J Radiat Oncol Biol Phys. 2010. 78: 404-8
9. Sarmiento JM, Wi MS, Piao Z, Stiner ES. Solitary cerebral metastasis from transitional cell carcinoma after a 14-year remission of urinary bladder cancer treated with gemcitabine: Case report and literature review. Surg Neurol Int. 2012. 3: 82-
10. Toschi L, Finocchiaro G, Bartolini S, Gioia V, Cappuzzo F. Role of gemcitabine in cancer therapy. Future Oncol. 2005. 1: 7-17
11. Tsugu A, Yoshiyama M, Matsumae M. Brain metastasis from large cell neuroendocrine carcinoma of the urinary bladder. Surg Neurol Int. 2011. 2: 84-
12. Xie J, Zhang XB, Wen J, Zhang YS, Li HZ. Comparison of clinicopathological features in metastatic upper tract urothelial carcinoma and urothelial bladder cancer. Int Urol Nephrol. 2016. 48: 481-7
13. Yasuda K, Nakamura M, Takamoto D, Sanjo H, Gohbara A, Teranishi JI. A case of long-term survivor after combined modality therapy for brain metastasis of bladder carcinoma. Hinyokika Kiyo. 2012. 58: 553-6
14. Yates DR, Catto JW. Distinct patterns and behaviour of urothelial carcinoma with respect to anatomical location: How molecular biomarkers can augment clinico-pathological predictors in upper urinary tract tumours. World J Urol. 2013. 31: 21-9