- Department of Neurologic Sciences, Division of Neurosurgery, Hospital de Base, Faculdade de Medicina de São José do Rio Preto, São Paulo, Brazil
- Department of Medicine, Universidade Federal de Sergipe, Aracaju, SE, Brazil
- Department of Medical Sciences, Division of Neurosurgery, School of Medicine, University Nove de Julho, São Paulo, Brazil
- Department of Cerebrovascular and Skull Base Surgery, Center of Neurology and Neurosurgery Associates, Hospital Beneficência Portuguesa de São Paulo, São Paulo, Brazil
- Department of Psychiatry and Medical Psychology, Faculdade de Medicina de São José do Rio Preto, São Paulo, Brazil
- Department of Neurologic Sciences, Division of Neurology, Hospital de Base, Faculdade de Medicina de São José do Rio Preto, São Paulo, Brazil
Correspondence Address:
Lucas Crociati Meguins
Department of Neurologic Sciences, Division of Neurology, Hospital de Base, Faculdade de Medicina de São José do Rio Preto, São Paulo, Brazil
DOI:10.4103/2152-7806.169552
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Meguins LC, Rodrigo Antônio Rocha da Cruz Adry, Sebastião Carlos da Silva Júnior, Pereira CU, Jean Gonçalves de Oliveira, de Morais DF, Gerardo Maria de Araújo Filho, Lúcia Helena Neves Marques. Microsurgical treatment of patients with refractory epilepsy and mesial temporal cavernous malformations: Clinical experience of a tertiary epilepsy center. Surg Neurol Int 16-Nov-2015;6:169
How to cite this URL: Meguins LC, Rodrigo Antônio Rocha da Cruz Adry, Sebastião Carlos da Silva Júnior, Pereira CU, Jean Gonçalves de Oliveira, de Morais DF, Gerardo Maria de Araújo Filho, Lúcia Helena Neves Marques. Microsurgical treatment of patients with refractory epilepsy and mesial temporal cavernous malformations: Clinical experience of a tertiary epilepsy center. Surg Neurol Int 16-Nov-2015;6:169. Available from: http://surgicalneurologyint.com/surgicalint_articles/microsurgical-treatment-of-patients-with-refractory-epilepsy-and/
Abstract
Background:Mesiotemporal cavernous malformation can occur in 10-20% of patients with cerebral cavernomas and are frequently associated with refractory.
Methods:A retrospective investigation was performed in the epilepsy clinic of a Brazilian tertiary referral epilepsy center, from January 2000 to March 2012.
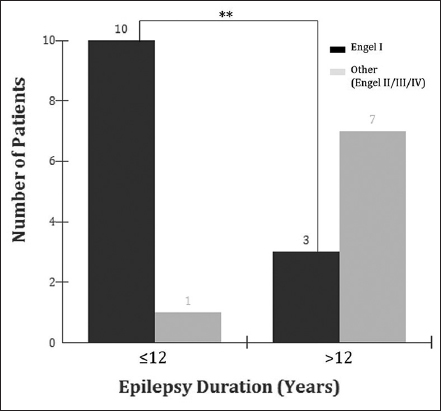
Results:A total of 21 patients were included in the study. Thirteen patients (62%) evolved to Engel I; 5 (24%) to Engel II, 2 (10%) to Engel III, and 1 (5%) to Engel IV. We observed that 10 (48%) patients with 12 years or less of epilepsy duration evolved to Engel I and 1 (5%) to Engel II; whereas from a total of 10 patients with epilepsy duration of more than 12 years, 3 (30%) evolved to Engel I and 7 (70%) to Engel II, III, or IV (P P1 ≠ P2).
Conclusion:Postsurgical seizure outcome for temporal lobe epilepsy associated with mesiotemporal cavernomas is very satisfactory.
Keywords: Cavernous malformations, mesiotemporal cavernous malformation, temporal lobe epilepsy
INTRODUCTION
Cerebral cavernous malformations are known to be highly epileptogenic lesions, but the underlying epileptogenic mechanisms are not fully understood. Asymptomatic microhemorrhages into the surrounding brain, with subsequent perifocal hemosiderosis and gliosis, are considered the major cause of seizure activity.[
METHODS
Study delineation
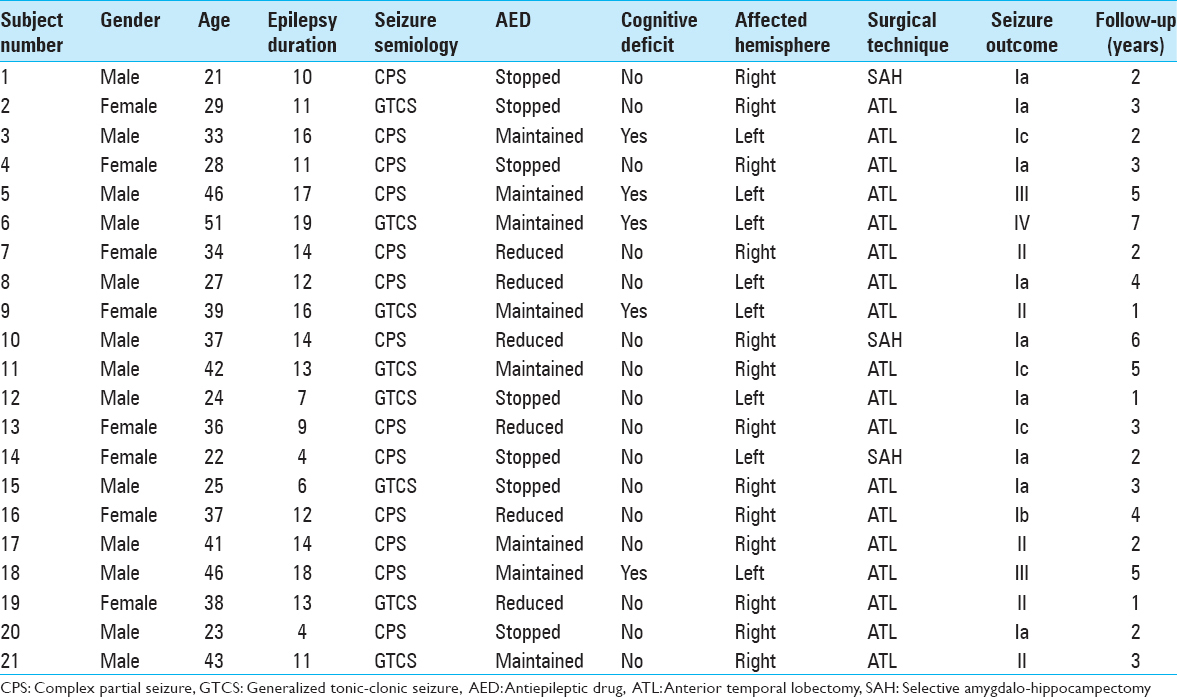
A retrospective observational investigation was conducted with data collection from all inpatients and outpatients treated in the epilepsy clinic of Faculdade de Medicina de Sao Jose do Rio Preto, a Brazilian tertiary referral epilepsy center, diagnosed with TLE-MTC from January 2000 to March 2012. Clinical data were obtained retrospectively from the patient records and files. For all patients with the diagnosis of MTC on magnetic resonance images (MRIs), the following data were collected: Gender, age at the surgery, handedness, type and number of antiepileptic drugs (AEDs) used, and results of formal neuropsychological evaluations. In addition, noninvasive video-electroencephalography (EEG) data and side of surgery were registered.
Presurgical evaluation
Patients were submitted to video-EEG monitoring using the Neuro Workbench software (Nihon Kohden Corporation) and Nihon Kohden hardware (Nihon Kohden Corporation) to record and later evaluated all the epileptic events. Every patient was analyzed by an experienced epileptologist as an integral part of inpatient assessment.
All patients were submitted to a neuropsychological assessment pre- and post-surgically (at 12 months). Verbal memory was assessed by a list of learning design, and figural memory by a design learning test using independent items. Memory deficits were defined as performance one standard deviation (SD) below of the normal performance of age-matched controls.
Brain MRI was obtained accordingly with specific epilepsy protocol using a 1.5 tesla scanner, Philips, at the Department of Neuroradiology in our institution. All MRIs were analyzed by an experienced neuroradiologist that confirmed the visual radiological diagnosis of mesial temporal lobe (parahippocampal gyrus, hippocampus, amigdalum, and uncus) cavernomas. Displaying the sagittal three-dimensional T1-weighted gradient-echo sequences, the next sequences were an axial and coronal fluid-attenuated inversion recovery fast spin-echo (section thickness, 3 mm), axial and coronal T2-weighted fast spin-echo (section thickness, 2 mm) and T1-weighted inversion recovery sequences (section thickness, 5 mm) [
Biopsy specimens were obtained from all patients with chronic drug-resistant and radiological evidence of MTC, who underwent surgical treatment. Surgical removal of the hippocampus was clinically indicated in every case, and all the patients were submitted to complete lobectomy. The standardized neuropathological analysis was performed in all the patients under this study. Surgical specimens submitted for neuropathological evaluation were microscopically analyzed by using hematoxylin and eosin staining. The pathologist reported their findings without the clinical or imaging data.
Outcome assessments and follow-up
Follow-up investigations were carried out in operated patients. At the 12 months follow-up, all the patients received a neurological examination including observation of behavior disorders, exploration of seizure outcome, and a cerebral 1.5 tesla MRI. Seizure outcome was classified as completely seizure-free since surgery, that is, Engel I, or not seizure-free (Engel II–IV).
Ethical statement
The Ethical Committee of our institution analyzed the project of the present study and approved the performance of our investigations. The study complies with the Declaration of Helsinki. Informed consent was taken from all patients and/or genitors.
Statistical analysis
Data collected from all the patients were organized in tables. Averages are expressed as the mean ± SD for parametric data and as median values for nonparametric data. Statistical analysis was performed utilizing the Fisher's exact test. A P < 0.01 was considered statistically significant.
RESULTS
Presurgical demographic and clinical characteristics
At the moment of the study, 533 patients underwent multidisciplinary investigation for epilepsy, and 21 (3.9%) patients fulfilled the inclusion criteria of radiological and pathological diagnosis of MTC.
Seizure control and follow-up
All the patients were followed during a minimum period of 1 year and a maximum of 7 years. The mean follow-up duration was of 3.14 ± 1.68 years. Three patients (14%) were followed during 1 year; 6 (29%) during 2 years; 5 (24%) during 3 years; 2 (10%) during 4 years; 2 (10%) during 5 years; 1 (5%) during 6 years; and 1 (5%) during 7 years. Seven patients (33%) stopped their AED; 6 (29%) reduced, and 8 (38%) maintained the AED use.
Seizure outcome following surgery revealed that 13 patients (62%) became Engel I, 5 (24%) to Engel II, 2 (10%) Engel III, and 1 (5%) Engel IV. We observed that 10 (48%) patients with 12 years or less of epilepsy duration evolved to Engel I and 1 (5%) to Engel II, whereas from a total of 10 patients with epilepsy duration of more than 12 years, 3 (30%) evolved to Engel I and 7 (70%) to Engel II, III, or IV (P < 0.001 [bilateral]; P1 ≠ P2) [
Postoperative neuropsychological assessment showed deterioration in 4 patients with the previous cognitive deficits. One patient recovered to its baseline. No patients with normal cognition preoperatively evolved memory deficits. No patients reported visual field defect after the surgery.
Complications
No operative death (30 days after surgery) was recorded. In the present investigation, 2 (9%) of patients evolved with infection of the surgical wound and were treated exclusively with oral antibiotics. No additional clinical complications where noted.
DISCUSSION
Cavernous brain malformations are vascular lesions with an estimated prevalence between 0.4% and 0.9%,[
In the present study, we presented our surgical series of 21 patients with refractory TLE-MTC. We found an average incidence of 1.61 case/year, affecting mainly man with age ranging from 30 to 40 years old. It was also noted that CPSs was the most common semiological feature, present in 62% of patients. Kivelev et al.,[
The present study also observed that patients suffering from epilepsy for a longer period might present a worse seizure outcome. In our analysis, 10 (48%) patients with 12 years or less of epilepsy duration evolved to Engel I and 1 (5%) to Engel II; whereas from the 8 patients with epilepsy duration of more than 12 years, 1 (5%) evolved to Engel I and 7 (33%) to Engel II, III, or IV (P = 0.0075 [bilateral]; P1 ≠ P2) [
There are several methodological aspects in the present findings, which should be interpreted in the context of a number of limitations. First, this study is a nonrandomized, retrospective investigation performed in a highly selected population of a tertiary epilepsy center. Second, these findings cannot be generalized for all types of TLE since the patients with dual pathology, or single disease other than MTC were excluded. On the other hand, the present study described the surgical outcomes of a relatively large number of patients that underwent surgery due to this uncommon pathology for a relatively extended follow-up duration.
CONCLUSION
The present study highlights that seizure outcome after respective epilepsy surgery for epileptogenic lesions, especially MTC, in adults is very satisfactory. However, surgical treatment should be considered early in the course of the disease to improve seizure control and to reduce suffering.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arita K, Kurisu K, Iida K, Hanaya R, Sugiyama K, Akimitsu T. Surgical treatment for intractable epilepsy caused by cavernous angioma in the temporal lobe of the dominant hemisphere – Three case reports. Neurol Med Chir (Tokyo). 2000. 40: 439-45
2. Aronica E, Leenstra S, van Veelen CW, van Rijen PC, Hulsebos TJ, Tersmette AC. Glioneuronal tumors and medically intractable epilepsy: A clinical study with long-term follow-up of seizure outcome after surgery. Epilepsy Res. 2001. 43: 179-91
3. Attar A, Ugur HC, Savas A, Yüceer N, Egemen N. Surgical treatment of intracranial cavernous angiomas. J Clin Neurosci. 2001. 8: 235-9
4. Bertalanffy H, Benes L, Miyazawa T, Alberti O, Siegel AM, Sure U. Cerebral cavernomas in the adult. Review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev. 2002. 25: 1-53
5. Boesebeck F, Janszky J, Kellinghaus C, May T, Ebner A. Presurgical seizure frequency and tumoral etiology predict the outcome after extratemporal epilepsy surgery. J Neurol. 2007. 254: 996-9
6. Bourgeois M, Di Rocco F, Sainte-Rose C. Lesionectomy in the pediatric age. Childs Nerv Syst. 2006. 22: 931-5
7. Cappabianca P, Alfieri A, Maiuri F, Mariniello G, Cirillo S, de Divitiis E. Supratentorial cavernous malformations and epilepsy: Seizure outcome after lesionectomy on a series of 35 patients. Clin Neurol Neurosurg. 1997. 99: 179-83
8. Chang EF, Gabriel RA, Potts MB, Garcia PA, Barbaro NM, Lawton MT. Seizure characteristics and control after microsurgical resection of supratentorial cerebral cavernous malformations. Neurosurgery. 2009. 65: 31-7
9. Cohen-Gadol AA, Wilhelmi BG, Collignon F, White JB, Britton JW, Cambier DM. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg. 2006. 104: 513-24
10. Elsharkawy AE, Alabbasi AH, Pannek H, Oppel F, Schulz R, Hoppe M. Long-term outcome after temporal lobe epilepsy surgery in 434 consecutive adult patients. J Neurosurg. 2009. 110: 1135-46
11. Englot DJ, Han SJ, Lawton MT, Chang EF. Predictors of seizure freedom in the surgical treatment of supratentorial cavernous malformations. J Neurosurg. 2011. 115: 1169-74
12. Hammen T, Romstöck J, Dörfler A, Kerling F, Buchfelder M, Stefan H. Prediction of postoperative outcome with special respect to removal of hemosiderin fringe: A study in patients with cavernous haemangiomas associated with symptomatic epilepsy. Seizure. 2007. 16: 248-53
13. Kivelev J, Niemelä M, Blomstedt G, Roivainen R, Lehecka M, Hernesniemi J. Microsurgical treatment of temporal lobe cavernomas. Acta Neurochir (Wien). 2011. 153: 261-70
14. Kivelev J, Niemelä M, Hernesniemi J. Characteristics of cavernomas of the brain and spine. J Clin Neurosci. 2012. 19: 643-8
15. Luyken C, Blümcke I, Fimmers R, Urbach H, Elger CE, Wiestler OD. The spectrum of long-term epilepsy-associated tumors: Long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia. 2003. 44: 822-30
16. Menzler K, Thiel P, Hermsen A, Chen X, Benes L, Miller D. The role of underlying structural cause for epilepsy classification: Clinical features and prognosis in mesial temporal lobe epilepsy caused by hippocampal sclerosis versus cavernoma. Epilepsia. 2011. 52: 707-11
17. Moran NF, Fish DR, Kitchen N, Shorvon S, Kendall BE, Stevens JM. Supratentorial cavernous haemangiomas and epilepsy: A review of the literature and case series. J Neurol Neurosurg Psychiatry. 1999. 66: 561-8
18. Rydenhag B, Flink R, Malmgren K. Surgical outcomes in patients with epileptogenic tumours and cavernomas in Sweden: Good seizure control but late referrals. J Neurol Neurosurg Psychiatry. 2013. 84: 49-53
19. Sommer B, Kasper BS, Coras R, Blumcke I, Hamer HM, Buchfelder M. Surgical management of epilepsy due to cerebral cavernomas using neuronavigation and intraoperative MR imaging. Neurol Res. 2013. 35: 1076-83
20. Steiger HJ, Markwalder TM, Reulen HJ. Clinicopathological relations of cerebral cavernous angiomas: Observations in eleven cases. Neurosurgery. 1987. 21: 879-84
21. von der Brelie C, Malter MP, Niehusmann P, Elger CE, von Lehe M, Schramm J. Surgical management and long-term seizure outcome after epilepsy surgery for different types of epilepsy associated with cerebral cavernous malformations. Epilepsia. 2013. 54: 1699-706
22. von der Brelie C, Schramm J. Cerebral cavernous malformations and intractable epilepsy: The limited usefulness of current literature. Acta Neurochir (Wien). 2011. 153: 249-59