- Department of Neurosurgery, Nara Medical University, Kashihara, Japan
- Department of Central Laboratory, Nara Medical University, Kashihara, Japan
- Ohnishi Neurological Centre, Akashi, Japan
Correspondence Address:
Yasushi Motoyama
Department of Neurosurgery, Nara Medical University, Kashihara, Japan
DOI:10.4103/2152-7806.173565
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Motoyama Y, Nakagawa I, Takatani T, Park H, Kotani Y, Tanaka Y, Gurung P, Park Y, Nakase H. Microvascular decompression for glossopharyngeal neuralgia using intraoperative neurophysiological monitoring: Technical case report. Surg Neurol Int 07-Jan-2016;7:
How to cite this URL: Motoyama Y, Nakagawa I, Takatani T, Park H, Kotani Y, Tanaka Y, Gurung P, Park Y, Nakase H. Microvascular decompression for glossopharyngeal neuralgia using intraoperative neurophysiological monitoring: Technical case report. Surg Neurol Int 07-Jan-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/microvascular-decompression-for-glossopharyngeal-neuralgia-using-intraoperative-neurophysiological-monitoring-technical-case-report/
Abstract
Background:Glossopharyngeal neuralgia (GN) is a rare functional disorder representing around 1% of cases of trigeminal neuralgia. Lancinating throat and ear pain while swallowing are the typical manifestations, and are initially treated using anticonvulsants such as carbamazepine. Medically refractory GN is treated surgically. Microvascular decompression (MVD) is reportedly effective against GN, superseding rhizotomy and tractotomy.
Methods:We encountered three patients with medically refractory GN who underwent MVD using intraoperative neurophysiological monitoring (IONM). The offending vessels were the posterior inferior cerebellar arteries, which were confirmed intraoperatively via a transcondylar fossa approach to be affecting the root exit zones of the glossopharyngeal and vagus nerves. As IONM, facial motor-evoked potentials (MEPs) and brainstem auditory-evoked potentials were monitored during microsurgery in all three patients. Pharyngeal and vagal MEPs were added for two patients to avoid postoperative dysphagia.
Results:GN disappeared immediately after surgery with complete preservation of hearing acuity and facial nerve function. Transient mild swallowing disturbance was observed in 1 patient without pharyngeal or vagal MEPs, whereas the remaining two patients with pharyngeal and vagal MEPs demonstrated no postoperative dysphagia.
Conclusion:Although control of severe pain is expected in surgical intervention for GN, lower cranial nerves are easily damaged because of their fragility, even in MVD. IONM including pharyngeal and vagal MEPs appears very useful for avoiding postoperative sequelae during MVD for GN.
Keywords: Glossopharyngeal neuralgia, intraoperative neurophysiological monitoring, lower cranial nerves, microvascular decompression
INTRODUCTION
Glossopharyngeal neuralgia (GN) consists of paroxysmal, transient, severe, sharp pain in the back of the throat, base of the tongue, tonsillar fossa, depth of the ear canal, and area beneath the angle of the jaw, all of which are territories innervated by the glossopharyngeal nerve and the pharyngeal branches of the vagus nerve. GN is therefore also called vagoglossopharyngeal neuralgia. Episodes usually last seconds to minutes and are often precipitated by chewing, coughing, yawning, talking, or swallowing.[
Neurophysiological monitoring
Facial, pharyngeal, and vagal MEPs
Following the induction of anesthesia with a short-acting agent for neuromuscular blockade, neuroanesthesia was maintained by intravenous infusion of propofol and remifentanil. Constant current stimuli consisting of four rectangular pulses with a 1.5-ms interstimulus interval were generated using a neuromaster MS-120B system (Nihon Kohden, Tokyo, Japan). Corkscrew electrodes were placed at positions C3 and C4 to stimulate transcranial MEPs for evaluation of not only the pyramidal tract but also the corticobulbar tract. The electrode placed on the other side of the target was used for anodal stimulation, because the cortex under the anode is preferentially stimulated. Facial MEPs were recorded from the orbicularis oculi and orbicularis oris muscles through paired stainless-steel needle electrodes inserted subdermally using a Neuromaster MEE-1000 system (Nihon Kohden). To obtain pharyngeal MEPs, paired stainless-steel needle electrodes were inserted directly after induction of anesthesia and intubation of an endotracheal tube into the stylopharyngeal muscle located in the posterior wall of the pharynx. Vagal MEPs were recorded from the false vocal cord using a surface electrode mounted on the endotracheal tube. To avoid large and overwriting stimulus artifacts, the band-pass filter was set at 100–3000 Hz. The amplitude of MEPs from the muscles innervated by the facial, glossopharyngeal, and vagus nerves was defined as the range between maximum positive and negative peaks of the polyphasic waveform. Basically, stimulation intensity was gradually increased until the amplitude of the target muscles could be elicited for the 1st time, which was defined as the threshold intensity of transcranial MEPs. Pharyngeal and vagal MEPs can be elicited by stimulation of either side due to the bilateral distribution of the corticobulbar tracts of the glossopharyngeal and vagus nerves. The intensity of stimulation for pharyngeal and vagus nerves was increased gradually until at least 10 μV of significant waveform with latency of approximately 10 ms was elicited; this point was defined as a threshold. The control amplitude was obtained by stimulation at an intensity of 20% above the threshold, representing supra-minimal stimulation.[
Brainstem auditory-evoked potentials
During surgery, potentials were recorded continuously by stimulation of the ear ipsilateral to the affected side with alternating rarefaction and condensation clicks. A stimulus rate of 17.1 Hz was used for optimal resolution of the collected peaks. A click intensity of at least a 110-dB sound pressure level was implemented, and white noise at an intensity of a 40-dB sound pressure level was used to mask activity in the contralateral ear. Five hundred trials were averaged over each 12-ms observation interval to obtain interpretable BAEP data. Three channels were used for recording. Channels 1 (Cz/A1) and 2 (Cz/A2) were formed between the vertex and mastoid process of the left and right ears, respectively. Channel 3 (Cz/Cv2) was spanned from the vertex to cervical vertebra C2. Wave V, the largest component of the BAEP waveform, was analyzed in real-time during the procedure. Significant changes in wave V were recorded and reported to the surgeon to monitor brainstem function, detect ischemia, and identify perturbations as indicators of hearing function. Two components of wave V were monitored and compared with baseline responses during the course of surgery: Amplitude and latency. Significant changes in each variable were recorded and reported to the surgeon. A significant change in amplitude took place when wave V decreased to 50% of the baseline value, and a significant change in latency occurred when wave V shifted at least 0.5 ms away from the baseline. In addition, the specified differences in latency or amplitude were required to occur during at least two consecutive trials in order to be considered significant and to prevent technical issues from interfering with data collection.
CASE ILLUSTRATIONS
Case 1
A 79-year-old man presented with a 5-year history of lancinating tongue, deep pharyngeal, and ear pain in the distribution of the glossopharyngeal nerve on the left side. Episodes of pain were triggered by talking, coughing, and especially swallowing, and interfered with his quality of life. GN was diagnosed and initially treated using CBZ, which achieved relief of severe throat pain. However, the efficacy of CBZ gradually decreased and severe throat pain recurred regardless of the maximum dose of medication. Other agents such as GPT, pregabalin (PGB), and diazepam (DZP) were tried, but provided no relief. The patient experienced debilitating generalized weakness and syncopal episodes with dose escalations. He was therefore referred to our department from neurology for consideration of surgical intervention after hospitalization for exacerbation of pain and symptoms related to the escalating doses of DZP and PGB. MVD was considered because MRI demonstrated an offending vessel compressing the root exit zone (REZ) of the glossopharyngeal nerve on the affected side. Surgery was performed via a transcondylar fossa approach using BAEPs and facial MEPs during the microsurgical procedure as IONM to minimize postoperative sequelae. The oribicularis oris and oculi muscles were used for recording transcranial MEPs for monitoring facial nerve function. The patient was placed in a park bench position while keeping the head slightly above the level of the heart to avoid venous engorgement. The head was rotated slightly to the right side, angled 30° toward the right shoulder. A unilateral hockey stick incision was performed. The skin together with the muscle was detached from the suboccipital surface. Lateral suboccipital craniotomy extending to the condylar fossa was performed and the dura mater was opened. The lateral medullary cistern was opened to expose the lower cranial nerves entering the jugular foramen. Arachnoid dissection and retraction of the cerebellum exposed the REZ on the ventral surface of the medulla oblongata. The offending vessel was confirmed to be a loop of the posterior inferior cerebellar artery (PICA) compressing the proximal sites of the glossopharyngeal and vagus nerves, which were also recognized under microscopy [
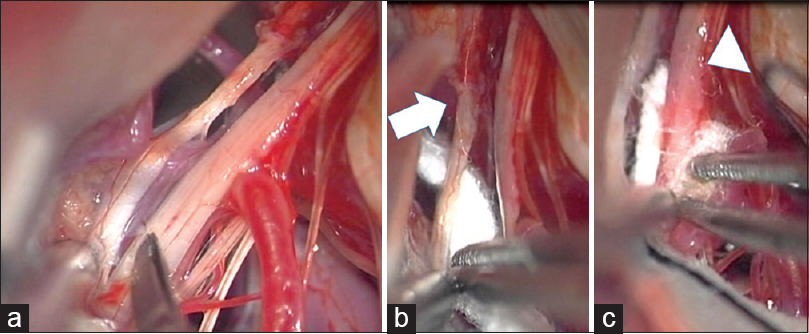
Figure 1
(a) Intraoperative view showing the posterior inferior cerebellar artery compressing the root exit zones of the glossopharyngeal and vagus nerves. (b) A piece of shredded Teflon is interposed between the root exit zone of IX nerve (arrow) and posterior inferior cerebellar artery. (c) Another piece of Teflon ball is inserted between the root exit zone of X nerve (arrow head) and posterior inferior cerebellar artery. IX: Glossopharyngeal nerve, X: Vagus nerve
Case 2
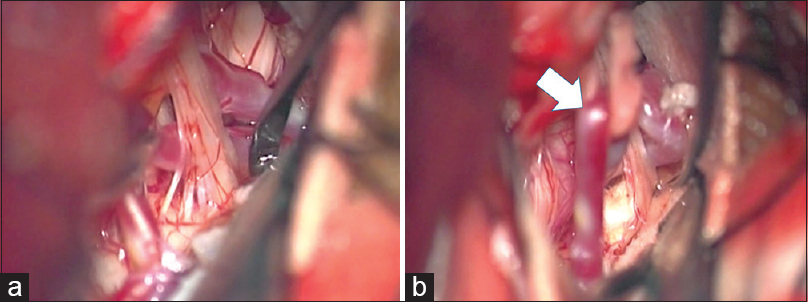
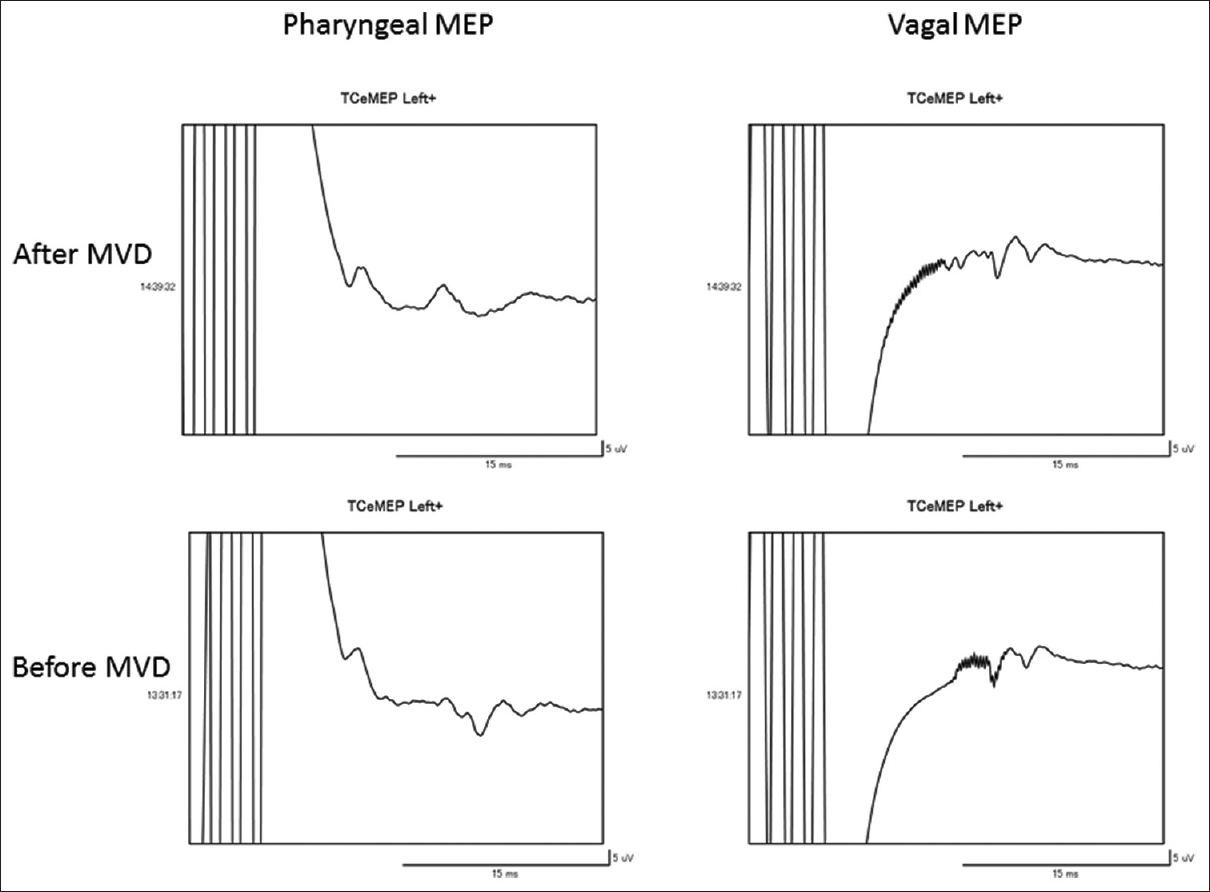
A 71-year-old woman had been suffering from left paroxysmal transient throat and ear pain, precipitated by drinking and eating. She underwent surgical intervention because CBZ provided insufficient relief of pain after several years of treatment. MRI showed a loop of the left PICA conflicting with the REZ of the glossopharyngeal nerve. She underwent MVD via a left condylar fossa approach in a park bench position. During the microscopic procedure, the loop of PICA was confirmed to be compressing the glossopharyngeal and vagus nerve fibers exiting the medulla oblongata [
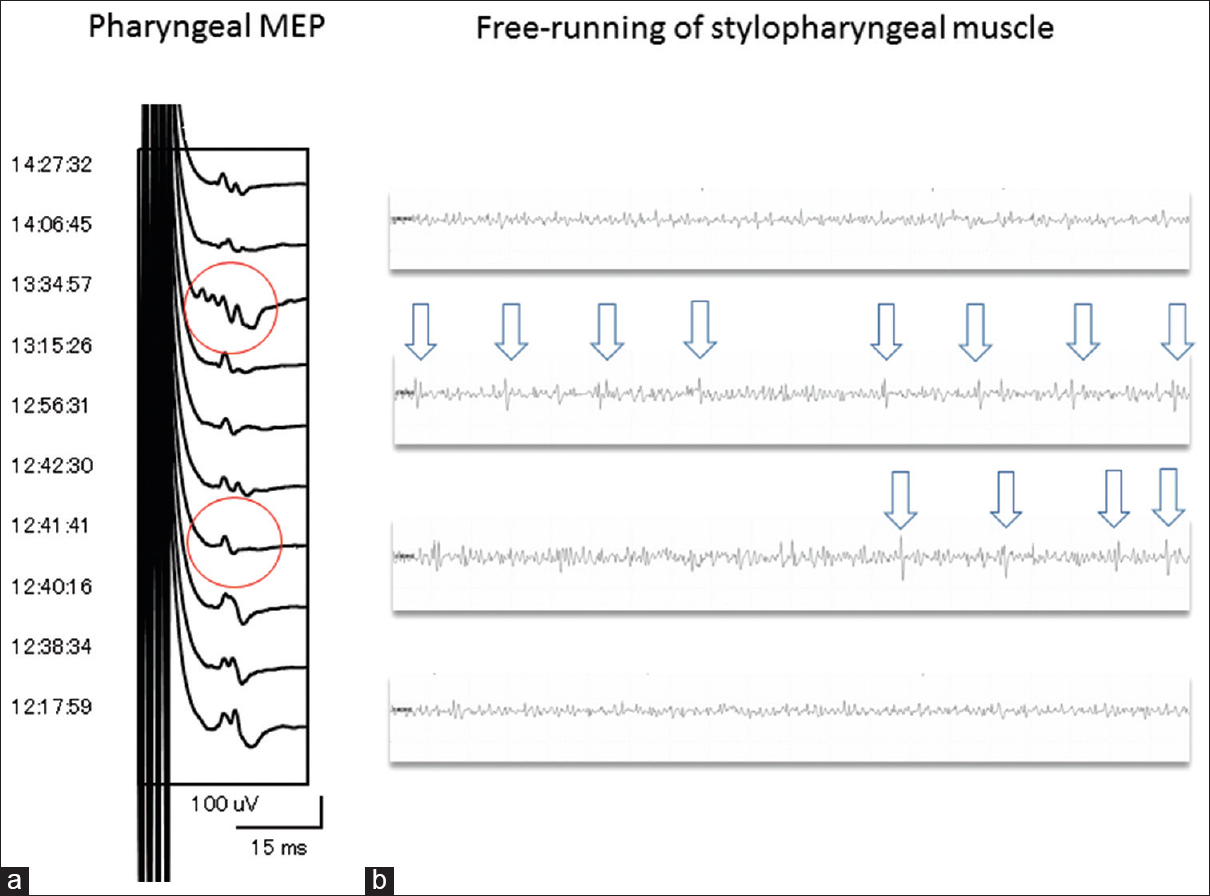
Figure 3
(a) Pharyngeal MEP elicited by transcranial electrical stimulation demonstrating transient reduction <50% of the amplitude of the control baseline and pleomorphic change (red-encircled) when free-running electromyography showed intermittent spike discharge sporadically. (b) Free-running electromyography of the stylopharyngeal muscle monitored continuously reveals intermittent spike discharges (white arrows) during direct procedure around the glossopharyngeal nerve
Case 3
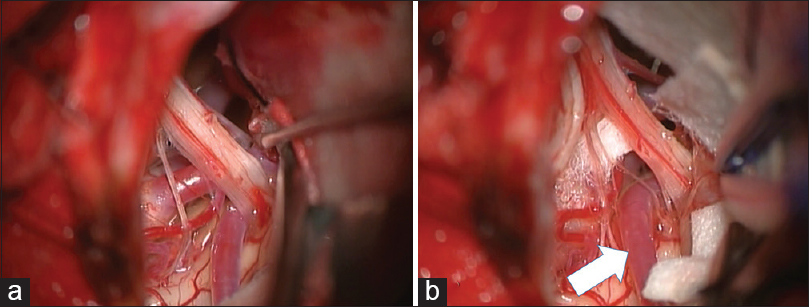
A 74-year-old man with a history of hypertension had started to feel intermittent serious throat pain in the left side while swallowing 4 years earlier. These symptoms had been treated using CBZ and PGN. Three years after initiation of medical therapy, recurrence of severe lancinating throat pain on the same side during swallowing proved refractory to CBZ pharmacotherapy. Other types of pharmacotherapy including DZP and trigeminal neuralgia were ineffective for the pain this time. MRI demonstrated the left PICA compressing the ventral side of the proximal glossopharyngeal nerve. Surgical intervention was performed using a transcondylar fossa approach with IONM, including BAEPs and facial, pharyngeal, and vagal MEPs. Lateral suboccipital craniotomy extending to the right condylar fossa was performed to obtain the operative trajectory in the inferolateral direction. The arachnoid membrane was dissected carefully and gentle upward retraction of the cerebellum exposed the cerebellomedullary cistern, where the glossopharyngeal and vagus nerves originate from the ventral surface of the medulla oblongata. The PICA proximal to the loop was observed to compress the ventral aspect of the glossopharyngeal and vagus nerve roots in the REZ [
RESULTS
Three patients with medically refractory GN were treated using MVD after MRI depicted the PICA offending lower cranial nerves. The PICA impinged on not only the glossopharyngeal nerve but also the vagus nerve, as confirmed intraoperatively and released by surgical devices. All three patients underwent surgery using a transcondylar fossa approach and neuralgia disappeared completely without any medical treatment postoperatively. One patient undergoing MVD without pharyngeal or vagal MEPs experienced mild, transient dysphagia postoperatively. The other two patients with lower cranial nerve monitoring did not demonstrate any sequelae after surgery, including dysphagia or dysphonia.
DISCUSSION
Among the surgical treatments for GN, MVD has been reported to be associated with a high success rate of long-term pain relief and a low rate of recurrence.[
The most frequent complication in MVD for GN is lower cranial nerve dysfunction, which is less likely than with other surgical interventions such as rhizotomy and radiofrequency thermocoagulopathy. The frequency of lower cranial nerve impairment ranges from 0% to 33.3% even according to literature limited to the last decade.[
Kawashima et al.[
Surface electrodes mounted on an endotracheal tube or laryngeal mask have been reported for recording EMG and CMAPs from the posterior wall of the pharynx and vocal cord.[
Free-running EMG is another important method for IONM, providing immediate feedback to the surgeon. The most important type of EMG recording during surgery is the neurotonic discharge, which comprises muscle unit potential activity in response to mechanical or metabolic irritation of the nerve innervating the monitored muscles.[
We adapted an alarm point of a 50% decrease in the amplitude of CMAPs from the stylopharyngeal muscle and false vocal cord elicited by transcranial electrical stimulation based on previous reports.[
Establishment of criteria for the evaluation of intraoperative impairment and prediction of outcomes for glossopharyngeal and vagus nerves represents an issue for the future.
Fukuda et al. reported that the amplitude of facial MEP after MVD for HFS was decreased in patients whose symptoms were resolved postoperatively, suggesting normalization of facial nerve excitability.[
CONCLUSION
IONM is useful during MVD for GN, facilitating the prevention of postoperative complications. CMAPs from the stylopharyngeal muscle and false vocal cord can be used in IONM of glossopharyngeal and vagus nerve functions. Transcranial pharyngeal and vagal MEP allow evaluation of the integrity of the relevant nerve functions, facilitating avoidance of dysphagia and hoarseness due to retraction of glossopharyngeal and vagus nerves after MVD for GN.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amagasaki K, Watanabe S, Naemura K, Nakaguchi H. Microvascular decompression for hemifacial spasm: How can we protect auditory function?. Br J Neurosurg. 2015. 29: 347-52
2. Chen J, Sindou M. Vago-glossopharyngeal neuralgia: A literature review of neurosurgical experience. Acta Neurochir (Wien). 2015. 157: 311-21
3. Ekbom KA, Westerberg CE. Carbamazepine in glossopharyngeal neuralgia. Arch Neurol. 1966. 14: 595-6
4. Esaki T, Osada H, Nakao Y, Yamamoto T, Maeda M, Miyazaki T. Surgical management for glossopharyngeal neuralgia associated with cardiac syncope: Two case reports. Br J Neurosurg. 2007. 21: 599-602
5. Ferroli P, Fioravanti A, Schiariti M, Tringali G, Franzini A, Calbucci F. Microvascular decompression for glossopharyngeal neuralgia: A long-term retrospectic review of the Milan-Bologna experience in 31 consecutive cases. Acta Neurochir (Wien). 2009. 151: 1245-50
6. Fukuda M, Oishi M, Hiraishi T, Fujii Y. Facial nerve motor-evoked potential monitoring during microvascular decompression for hemifacial spasm. J Neurol Neurosurg Psychiatry. 2010. 81: 519-23
7. Fukuda M, Oishi M, Hiraishi T, Saito A, Fujii Y. Pharyngeal motor evoked potentials elicited by transcranial electrical stimulation for intraoperative monitoring during skull base surgery. J Neurosurg. 2012. 116: 605-10
8. García-Callejo FJ, Velert-Vila MM, Talamantes-Escribá F, Blay-Galaud L. Clinical response of gabapentin for glossopharyngeal neuralgia. Rev Neurol. 1999. 28: 380-4
9. Gaul C, Hastreiter P, Duncker A, Naraghi R. Diagnosis and neurosurgical treatment of glossopharyngeal neuralgia: Clinical findings and 3-D visualization of neurovascular compression in 19 consecutive patients. J Headache Pain. 2011. 12: 527-34
10. Giorgi C, Broggi G. Surgical treatment of glossopharyngeal neuralgia and pain from cancer of the nasopharynx.A 20-year experience. J Neurosurg. 1984. 61: 952-5
11. Habeych ME, Crammond DJ, Gardner P, Thirumala PD, Horowitz MB, Balzer JR. Intraoperative neurophysiological monitoring of microvascular decompression for glossopharyngeal neuralgia. J Clin Neurophysiol. 2014. 31: 337-43
12. Harper CM, Daube JR. Facial nerve electromyography and other cranial nerve monitoring. J Clin Neurophysiol. 1998. 15: 206-16
13. Holland NR. Intraoperative electromyography. J Clin Neurophysiol. 2002. 19: 444-53
14. Husain AM, Wright DR, Stolp BW, Friedman AH, Keifer JC. Neurophysiological intraoperative monitoring of the glossopharyngeal nerve: Technical case report. Neurosurgery. 2008. 63: 277-8
15. Ito E, Ichikawa M, Itakura T, Ando H, Matsumoto Y, Oda K. Motor evoked potential monitoring of the vagus nerve with transcranial electrical stimulation during skull base surgeries. J Neurosurg. 2013. 118: 195-201
16. Kandan SR, Khan S, Jeyaretna DS, Lhatoo S, Patel NK, Coakham HB. Neuralgia of the glossopharyngeal and vagal nerves: Long-term outcome following surgical treatment and literature review. Br J Neurosurg. 2010. 24: 441-6
17. Katusic S, Williams DB, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of glossopharyngeal neuralgia, Rochester, Minnesota, 1945-1984. Neuroepidemiology. 1991. 10: 266-75
18. Kaul AK, Chawla TN, Chandra S, Dave VS. Clinical trial of carbamazepine in the management of trigeminal and glossopharyngeal neuralgia. J Indian Dent Assoc. 1973. 45: 8-13
19. Kawashima M, Matsushima T, Inoue T, Mineta T, Masuoka J, Hirakawa N. Microvascular decompression for glossopharyngeal neuralgia through the transcondylar fossa (supracondylar transjugular tubercle) approach. Neurosurgery. 2010. 66: 275-80
20. Laha RK, Jannetta PJ. Glossopharyngeal neuralgia. J Neurosurg. 1977. 47: 316-20
21. Leal PR, Hermier M, Froment JC, Souza MA, Cristino-Filho G, Sindou M. Preoperative demonstration of the neurovascular compression characteristics with special emphasis on the degree of compression, using high-resolution magnetic resonance imaging: A prospective study, with comparison to surgical findings, in 100 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Acta Neurochir (Wien). 2010. 152: 817-25
22. Ma Z, Li M, Cao Y, Chen X. Keyhole microsurgery for trigeminal neuralgia, hemifacial spasm and glossopharyngeal neuralgia. Eur Arch Otorhinolaryngol. 2010. 267: 449-54
23. Martínez-González JM, Martínez-Rodríguez N, Calvo-Guirado JL, Brinkmann JC, Dorado CB. Glossopharyngeal neuralgia: A presentation of 14 cases. J Oral Maxillofac Surg. 2011. 69: e38-41
24. Motoyama Y, Kawaguchi M, Yamada S, Nakagawa I, Nishimura F, Hironaka Y. Evaluation of combined use of transcranial and direct cortical motor evoked potential monitoring during unruptured aneurysm surgery. Neurol Med Chir (Tokyo). 2011. 51: 15-22
25. Munch TN, Rochat P, Astrup J. Vagoglossopharyngeal neuralgia treated with vascular decompression. Ugeskr Laeger. 2009. 171: 2654-5
26. Patel A, Kassam A, Horowitz M, Chang YF. Microvascular decompression in the management of glossopharyngeal neuralgia: Analysis of 217 cases. Neurosurgery. 2002. 50: 705-10
27. Sharma N, Mishra D. International classification of headache disorders, 3rd edition: What the pediatrician needs to know. Indian Pediatr. 2014. 51: 123-4
28. Sindou M, Keravel Y. Neurosurgical treatment of vago-glossopharyngeal neuralgia. Neurochirurgie. 2009. 55: 231-5
29. Thirumala P, Meigh K, Dasyam N, Shankar P, Sarma KR, Sarma DR. The incidence of high-frequency hearing loss after microvascular decompression for trigeminal neuralgia, glossopharyngeal neuralgia, or geniculate neuralgia. J Neurosurg. 2015. 123: 1500-6
30. Thirumala PD, Carnovale G, Habeych ME, Crammond DJ, Balzer JR. Diagnostic accuracy of brainstem auditory evoked potentials during microvascular decompression. Neurology. 2014. 83: 1747-52
31. Wang YN, Zhong J, Zhu J, Dou NN, Xia L, Visocchi M. Microvascular decompression in patients with coexistent trigeminal neuralgia, hemifacial spasm and glossopharyngeal neuralgia. Acta Neurochir (Wien). 2014. 156: 1167-71
32. Xiong NX, Zhao HY, Zhang FC, Liu RE. Vagoglossopharyngeal neuralgia treated by microvascular decompression and glossopharyngeal rhizotomy: Clinical results of 21 cases. Stereotact Funct Neurosurg. 2012. 90: 45-50
33. Yang CP, Nagaswami S. Cardiac syncope secondary to glossopharyngeal neuralgia – Effectively treated with carbamazepine. J Clin Psychiatry. 1978. 39: 776-8