- Department of Neurosurgery, Fuji Brain Institute and Hospital, Fujinomiya, Shizuoka, Japan
- Department of Neurosurgery, NTT Medical Center Tokyo, Tokyo, Japan
- Department of Neurosurgery, The University of Tokyo Hospital, Tokyo, Japan
Correspondence Address:
Hideaki Ono
Department of Neurosurgery, The University of Tokyo Hospital, Tokyo, Japan
DOI:10.4103/sni.sni_154_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hideaki Ono, Tomohiro Inoue, Shinya Suematsu, Takeo Tanishima, Akira Tamura, Isamu Saito, Nobuhito Saito. Middle cerebral artery dissection causing subarachnoid hemorrhage and cerebral infarction: Trapping with high-flow bypass preserving the lenticulostriate artery. 25-Jul-2017;8:157
How to cite this URL: Hideaki Ono, Tomohiro Inoue, Shinya Suematsu, Takeo Tanishima, Akira Tamura, Isamu Saito, Nobuhito Saito. Middle cerebral artery dissection causing subarachnoid hemorrhage and cerebral infarction: Trapping with high-flow bypass preserving the lenticulostriate artery. 25-Jul-2017;8:157. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8503
Abstract
Background:Spontaneous intracranial arterial dissection (IAD) is an increasingly important cause of stroke, such as subarachnoid hemorrhage (SAH) and hemodynamic or thromboembolic cerebral ischemia. IAD usually occurs in the posterior circulation, and is relatively rare in the anterior circulation including the middle cerebral artery (MCA). Various surgical and endovascular methods to reduce blood flow in the dissected lesion have been proposed, but no optimum treatment has been established.
Case Description:An 80-year-old woman with dissection in the M1 portion of the MCA manifesting as SAH presented with repeated hemorrhage and cerebral infarction in the area of the inferior trunk of the MCA. High-flow bypass to the MCA was performed and the dissecting lesion was trapped. Prevention of repeated hemorrhage was achieved, and blood flow was preserved to the lenticulostriate artery as well as the MCA area distal to the lesion.
Conclusions:Treatment strategy for IAD of the MCA should be planned for each patient and condition, and surgery should be performed promptly to prevent critical rebleeding given the high recurrence rate. In addition, preventing re-rupture of the IAD, and preserving important perforators around the lesion and blood flow distal to the dissection should be targeted by the treatment strategy.
Keywords: Bypass, intracranial arterial dissection, lenticulostriate artery, middle cerebral artery, trap
INTRODUCTION
Spontaneous intracranial arterial dissection (IAD) has become increasingly important as a cause of stroke, such as subarachnoid hemorrhage (SAH) and hemodynamic or thromboembolic cerebral ischemia, with the development of diagnostic imaging methods.[
We describe a case of IAD in the MCA treated with trapping of the dissecting lesion and high-flow bypass using a radial artery graft (RAG) to the MCA, which preserved an important perforating artery, the lenticulostriate artery (LSA).
CASE REPORT
History
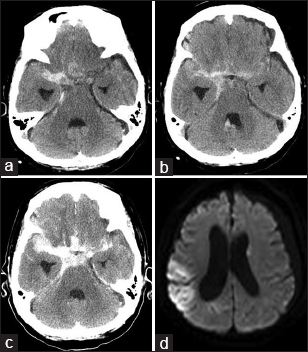
An 80-year-old woman with a past medical history of hypertension, diabetes mellitus, dyslipidemia, renal failure, angina pectoris, and dementia presented with headache and decline in cognitive function persisting for 3 days. Head computed tomography (CT) at a local hospital disclosed SAH, and she was referred to our hospital at night. On admission, her Glasgow Coma Scale score was 14, and CT demonstrated SAH, with a thick hematoma in the right sylvian cistern [
Figure 2
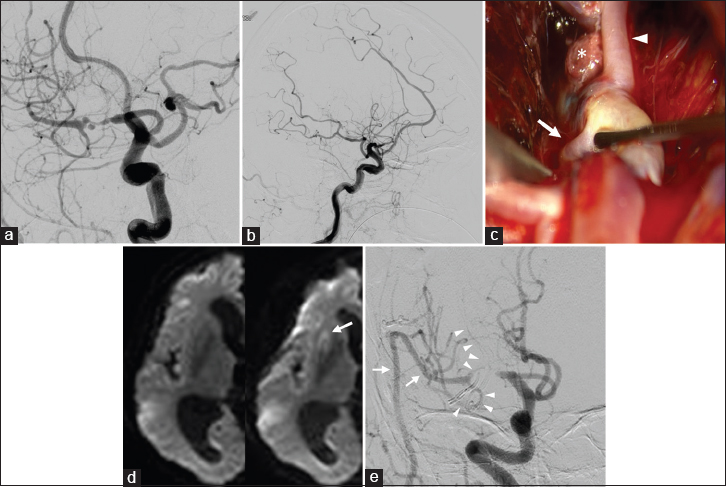
Preoperative DSA, left anterior oblique (a) and lateral (b) projections, showing severe stenosis and irregular aneurysmal dilatation in the M1 portion of the middle cerebral artery (MCA), and poor perfusion of the MCA territory compared with the anterior and posterior cerebral artery areas. Dissecting aneurysm with a purplish-red wall arising from the M1 trunk was found at surgery, just proximal to the lenticulostriate artery (LSA, arrow) and inferior trunk (arrowhead) of the MCA, part of the vessel wall was broken, and thrombus (asterisk) had protruded (c). Postoperative diffusion-weighted image revealing a new small infarction in the anterolateral part of the putamen (d, arrow), and DSA showing good patency of the radial artery graft (arrows), LSA, and inferior trunk of the MCA (arrowheads), and no flow to the dissecting lesion (e)
On the day after admission, CT before surgery identified increased hematoma, indicating repeated hemorrhage, and diffusion-weighted imaging revealed infarctions mainly in the right MCA area [
Operation
Neuroanesthesia was induced under monitoring of somatosensory evoked potentials (SSEPs) of the left extremities and motor evoked potentials (MEPs) of the left upper limb. A curvilinear frontotemporal skin incision was made to expose the right cervical carotid bifurcation, and the superficial temporal artery (STA) was meticulously prepared under the operating microscope. The RAG was harvested concurrently by another neurosurgeon. Frontotemporal craniotomy was performed, and a subzygomatic tunnel was made for the RAG. The sylvian fissure was split under the operating microscope, the hematoma was irrigated and removed carefully, and the M2 and M3 portions of the MCA were exposed. First, a back-up STA-M3 bypass was made distal to the M2 portion for RAG anastomosis. Then, the harvested RAG was gently pulled through the subzygomatic tunnel, that is, between the lateral pterygoid muscle and the temporal muscle from the cranium to the neck through the lateral corridor of the stylohyoid muscle and the posterior belly of the digastric muscle toward the external carotid artery (ECA).[
Postoperative course
Postoperative CT detected no increase in hemorrhage. Postoperative magnetic resonance angiography showed good patency of the bypass, and diffusion-weighted imaging revealed a new small infarction in the anterolateral part of the putamen [
DISCUSSION
Patients with IAD causing SAH are usually treated with surgical or endovascular procedures because the mortality for patients with IAD and SAH ranges between 19% and 50%, and the recurrence rate of SAH is reported as high as up to 40%.[
Three previous cases have illustrated in detail the surgical treatment of SAH caused by dissection of the M1 portion of the MCA.[
In our present case, the ruptured dissection was located in the M1 portion of the MCA, near the ATA, LSA, and inferior trunk of the MCA, and repeated hemorrhage occurred. In addition, cerebral infarction of the MCA inferior trunk area developed before surgery. We assumed the cause was thromboembolism originating from the dissection, because a hemodynamic cause would have limited the infarction area to the inferior trunk of the MCA. We had to plan measures for preventing rebleeding and thromboembolic infarction in the early phase given the high risk of repeated hemorrhage and infarction. The optimal treatment method in this case needed to consider three points as follows: how to prevent repeated hemorrhage, how to prevent thromboembolic infarction, and how to avoid cerebral infarction in the perforating arteries from the M1 portion as well as perfusion areas of the distal MCA. First, no flow into the dissection should be allowed to prevent hemorrhage, because this patient suffered repeated hemorrhage after admission. Second, the thrombus in the dissecting lumen should be fixed in place to prevent migration to distal portions of the MCA. Reconstructive methods aiming to maintain the parent artery are not suitable for these needs, whereas deconstructive trapping of the dissecting lesion might be the best option. Third, the appropriate flow out design would be critical to maintain blood flow and to avoid late thrombosis in the nearby important perforator, the LSA,[
Fortunately in this case, the LSA originated just distal to the dissecting lesion. If the LSA had been involved in the lesion, deconstructive methods such as trapping would have sacrificed the LSA, and reconstructive treatment such as aneurysm sac clipping or stenting would have been necessary. We considered that low-flow bypass was ideal if that would perfuse areas of middle cerebral artery adequately through the term of vasospasm after subarachnoid hemorrhage, because the patient was old and had variable medical complications.
To examine whether the low-flow bypass would replace M1 substantially or not, meticulous collateral evaluation by balloon occlusion test, which would be a predictive and reliable test, should have been performed, but we could not perform due to the unavailability of an endovascular team in our institution. Even with endovascular expertise, we would have avoided balloon occlusion test because of the embolic risk related to the dissecting lesion already causing infarction. In addition, the long-term cerebral ischemic risk would not be predictable. For these reasons, we could not get evidence that low-flow bypass would replace M1 substantially in the future, and we considered that ECA-RAG-M2 bypass with appropriate outflow design might provide sufficient flow to the MCA area and perforating artery of M1 to counteract the abrupt therapeutic M1 occlusion and continue through the term of vasospasm after SAH. Therefore, we planned to form the ECA-RAG-M2 bypass followed by trapping of the dissecting lesion between the inferior trunk of the M2 and ATA, so that the bypass flow would perfuse the MCA territory and LSA, and flow out to the inferior trunk of the M2, with the anterograde flow from the ICA passing to the ATA, and no flow in the dissection [
Figure 3
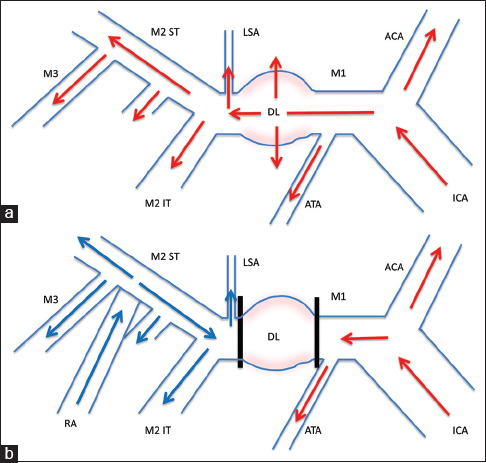
Schematic drawing of the surgery. Preoperative (a) and postoperative (b) hemodynamics are shown. Preoperatively, the internal carotid artery (ICA) supplied all of the anterior cerebral artery (ACA) and middle cerebral artery (MCA) territories, including the dissecting lesion (DL) with risk of rebleeding. With construction of the external carotid artery (ECA)-radial artery (RA)-M2 bypass and trapping of the DL in M1, the ICA would perfuse the ACA and anterior temporal artery (ATA) territories, and the ECA-RA-M2 bypass would supply the MCA territory distal to the lesion including the lenticulostriate artery (LSA), with no blood flow into the dissection. Red arrows: blood flow from ICA, blue arrows: blood flow from ECA-RA-M2 bypass, IT inferior trunk, ST superior trunk
We made STA-M3 bypass for insurance during temporary clamping of following anastomosis between RAG and M2. Although this supportive anastomosis increases the total time of the operation, it is useful for avoiding major ischemic injury during large-caliber anastomosis because it is impossible to know how long the recipient M2 can endure temporary occlusion during anastomosis.[
Surgery was performed successfully as planned, and prevented both repeated hemorrhage and cerebral infarction in the MCA territory distal to the dissection. Flow to the LSA and M2 inferior trunk was supplied from the bypass as shown by postoperative DSA. New infarction occurred in the anterolateral part of the putamen, which may be the territory of the lateral LSA,[
Treatment strategy for IAD of the MCA should be planned for each patient and condition, and surgery should be performed promptly to prevent critical rebleeding after SAH considering the high recurrence rate. In addition, preventing rerupture of the IAD and preserving important perforators around the lesion and blood flow distal to the dissection should be targets of the treatment strategy.
Financial support and sponsorship
Nil.
Conflicts of interest/disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
Video available on: www.surgicalneurologyint.com
References
1. Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015. 14: 640-54
2. Feekes JA, Cassell MD. The vascular supply of the functional compartments of the human striatum. Brain. 2006. 129: 2189-201
3. Hasegawa H, Inoue T, Tamura A, Saito I. Tailored flow sequestration treatment using high-flow and low-flow bypass for partially thrombosed giant internal carotid artery aneurysm-a technical case report. Neurosurg Rev. 2016. 39: 699-705
4. Hashimoto H, Iida J, Shin Y, Hironaka Y, Sakaki T. Subarachnoid hemorrhage from intracranial dissecting aneurysms of the anterior circulation. Two case reports. Neurol Med Chir (Tokyo). 1999. 39: 442-6
5. Hongo K, Horiuchi T, Nitta J, Tanaka Y, Tada T, Kobayashi S. Double-insurance bypass for internal carotid artery aneurysm surgery. Neurosurgery. 2003. 52: 597-602
6. Ishikawa T, Kamiyama H, Kobayashi N, Tanikawa R, Takizawa K, Kazumata K. Experience from “double-insurance bypass.” Surgical results and additional techniques to achieve complex aneurysm surgery in a safer manner. Surg Neurol. 2005. 63: 485-90
7. Ishishita Y, Tanikawa R, Noda K, Kubota H, Izumi N, Katsuno M. Universal extracranial-intracranial graft bypass for large or giant internal carotid aneurysms: Techniques and results in 38 consecutive patients. World Neurosurg. 2014. 82: 130-9
8. Kalani MY, Zabramski JM, Hu YC, Spetzler RF. Extracranial-intracranial bypass and vessel occlusion for the treatment of unclippable giant middle cerebral artery aneurysms. Neurosurgery. 2013. 72: 428-35
9. Mizutani T. Subarachnoid hemorrhage associated with angiographic “stenotic” or “occlusive” lesions in the carotid circulation. Surg Neurol. 1998. 49: 495-503
10. Mizutani T. Natural course of intracranial arterial dissections. J Neurosurg. 2011. 114: 1037-44
11. Murakami K, Shimizu H, Matsumoto Y, Tominaga T. Acute ischemic complications after therapeutic parent artery occlusion with revascularization for complex internal carotid artery aneurysms. Surg Neurol. 2009. 71: 434-41
12. Niikawa S, Yamada J, Sumi Y, Yamakawa H. Dissecting aneurysm of the middle cerebral artery manifesting as subarachnoid hemorrhage and hemorrhagic infarctions-case report. Neurol Med Chir (Tokyo). 2002. 42: 62-6
13. Ohkuma H, Suzuki S, Ogane K. Study Group of the Association of Cerebrovascular Disease in Tohoku J. Dissecting aneurysms of intracranial carotid circulation. Stroke. 2002. 33: 941-7
14. Ono H, Nakatomi H, Tsutsumi K, Inoue T, Teraoka A, Yoshimoto Y. Symptomatic recurrence of intracranial arterial dissections: Follow-up study of 143 consecutive cases and pathological investigation. Stroke. 2013. 44: 126-31
15. Peron S, Jimenez-Roldan L, Cicuendez M, Millan JM, Ricoy JR, Lobato RD. Ruptured dissecting cerebral aneurysms in young people: Report of three cases. Acta Neurochir (Wien). 2010. 152: 1511-7
16. Sakamoto S, Ikawa F, Kawamoto H, Ohbayashi N, Inagawa T. Acute surgery for ruptured dissecting aneurysm of the M3 portion of the middle cerebral artery. Neurol Med Chir (Tokyo). 2003. 43: 188-91
17. Sato K, Yamada M, Abe K, Oka H, Kurata A, Fujii K. Tailored flow alteration treatment for intracranial internal carotid artery aneurysms: Strategy beyond parent artery occlusion with bypass. Case report. Neurol Med Chir (Tokyo). 2012. 52: 213-6
18. Yamaura A, Ono J, Hirai S. Clinical picture of intracranial non-traumatic dissecting aneurysm. Neuropathology. 2000. 20: 85-90
19. Yonekawa Y, Zumofen D, Imhof HG, Roth P, Khan N. Hemorrhagic cerebral dissecting aneurysms: Surgical treatments and results. Acta Neurochir Suppl. 2008. 103: 61-9