- Department of Neurosurgery, Kumamoto General Hospital, Yatsushiro, Kumamoto, Japan.
- Department of Neurosurgery, School of Medicine, Kurume University, Kurume, Fukuoka, Japan.
- Department of Neurosurgery, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan.
Correspondence Address:
Hirotaka Inoue
Department of Neurosurgery, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan.
DOI:10.25259/SNI_249_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hirotaka Inoue1, Takayuki Kawano2, Yasuyuki Kaku3, Akitake Mukasa3. Minimally invasive treatment strategy for partially thrombosed anterior inferior cerebellar artery aneurysm: A case report. 26-Apr-2021;12:195
How to cite this URL: Hirotaka Inoue1, Takayuki Kawano2, Yasuyuki Kaku3, Akitake Mukasa3. Minimally invasive treatment strategy for partially thrombosed anterior inferior cerebellar artery aneurysm: A case report. 26-Apr-2021;12:195. Available from: https://surgicalneurologyint.com/surgicalint-articles/10748/
Abstract
Background: Partially thrombosed anterior inferior cerebellar artery (AICA) aneurysms are extremely rare; thus, no established therapeutic approach exists.
Case Description: We report a large, partially thrombosed AICA aneurysm and discuss its therapeutic nuances. The aneurysm was asymptomatic; therefore, we aimed to treat it through a minimally invasive procedure. The aneurysm was of fusiform type and the proximal neck of the aneurysm was positioned at midline in front of the brainstem. To approach the neck, posterior transpetrosal approach is recommended. However, this approach can be invasive; thus, we performed distal clipping of the aneurysm using transcondylar fossa approach with occipital artery-AICA bypass to avoid ischemia of the AICA territory. Although the size of the aneurysm initially increased, it subsequently decreased.
Conclusion: This is a rare case report describing the long-term clinical course after distal clipping in detail. We showed that traditional microsurgical techniques can be applied to treat patients with new, minimally invasive treatment strategies.
Keywords: Anterior inferior cerebellar artery, Distal clipping, Flow alteration, Partially thrombosed aneurysm
INTRODUCTION
Anterior inferior cerebellar artery (AICA) aneurysms are uncommon, accounting for <1% of all intracranial aneurysms. Proximal AICA aneurysms are even rarer.[
For a better understanding of this rare clinical entity, we present our report on a large, partially thrombosed AICA aneurysm and discuss the therapeutic nuances.
CASE DESCRIPTION
A 68-year-old woman presented with dizziness and headache. She had no neurological deficits, such as paralysis or sensory disturbances. Magnetic resonance imaging (MRI) revealed a 9 mm, unruptured, thrombosed aneurysm anterior to the brainstem [
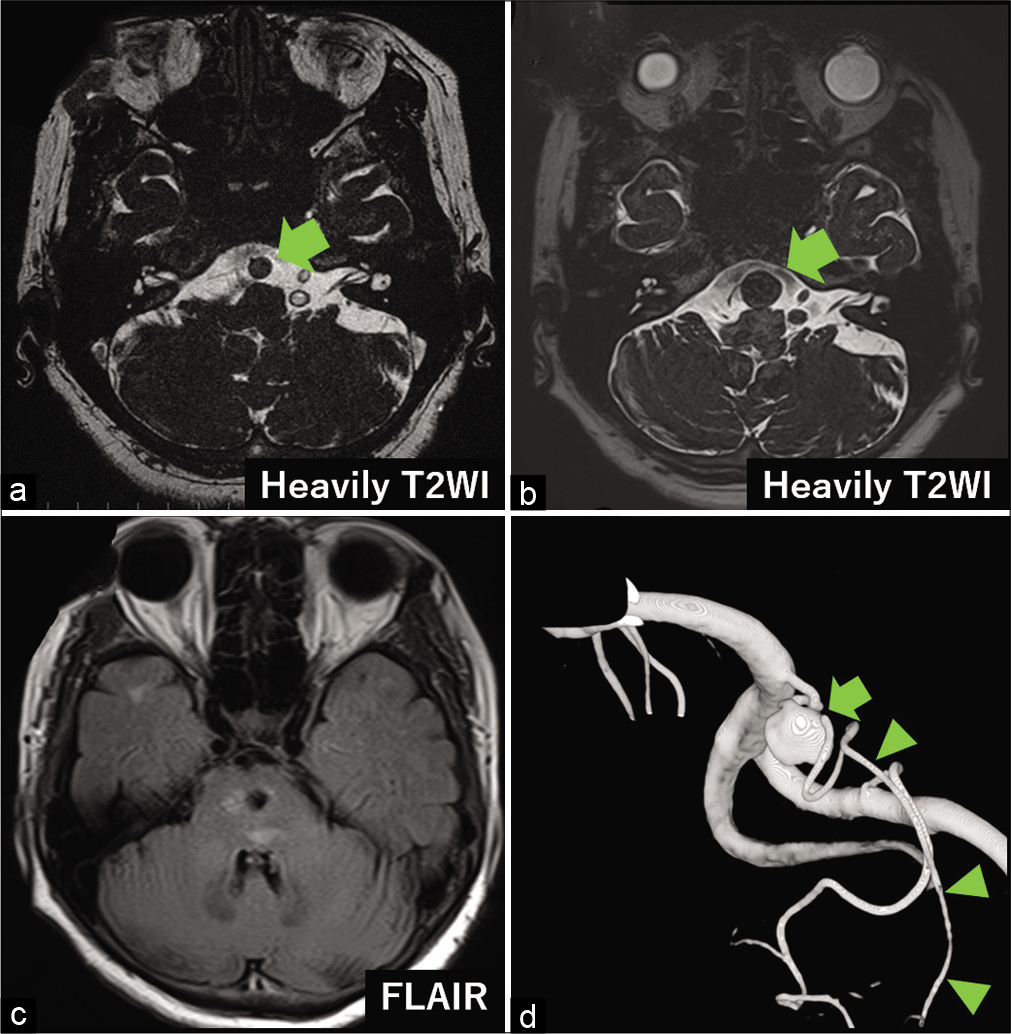
Figure 1:
Imaging findings before and after the treatment. (a) Magnetic resonance (MR) image showing the aneurysm (9 mm, arrow) anterior to the brainstem when the patient was 68 years old, (b) MR image showing enlarged aneurysm (14 mm) when the patient was 70 years old (arrow), (c) MR image showing brainstem mass effect due to the aneurysm, (d) three-dimensional digital subtraction angiography showing a right proximal anterior inferior cerebellar artery (AICA) aneurysm (arrow) and the distal AICA (arrowheads) appearing from the aneurysm body.
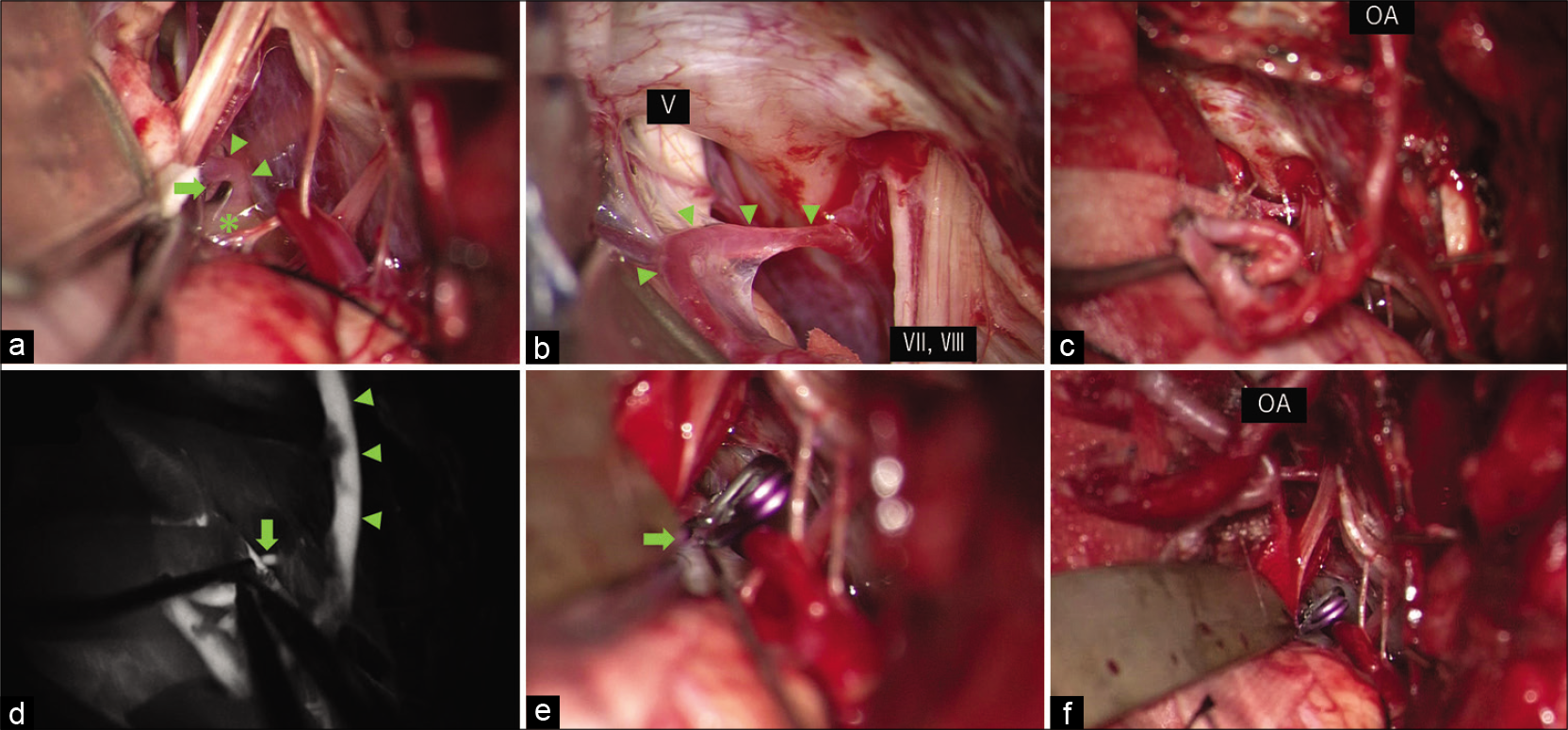
Figure 2:
Intraoperative findings. (a) Intraoperative photograph showing a right proximal anterior inferior cerebellar artery (AICA) aneurysm (asterisk), the distal AICA (arrowheads) appearing from the aneurysm body, and the branch vessel (arrow) appearing from AICA, (b) intraoperative photograph showing a distal part of the AICA (arrowheads) suitable for bypass, (c) intraoperative photograph showing occipital artery AICA bypass, (d) indocyanine green video angiography showing the adequate flow of the bypass. Retrograde filling of AICA was observed (arrow), (e) intraoperative photograph showing clipping of the AICA just distal to the aneurysm. Small branch from AICA was preserved (arrow), (f) intraoperative photograph showing the panoramic view of the surgical field after the bypass and the clipping.
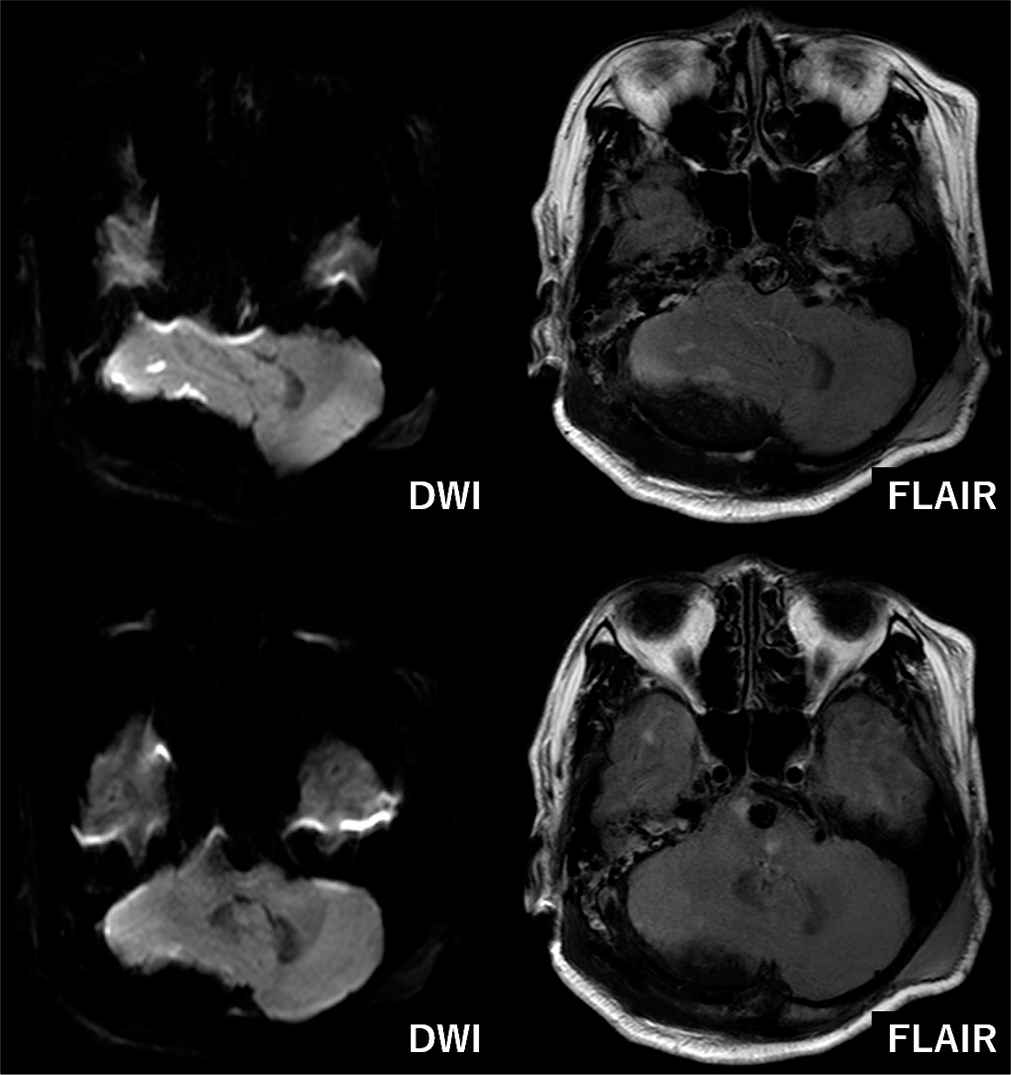
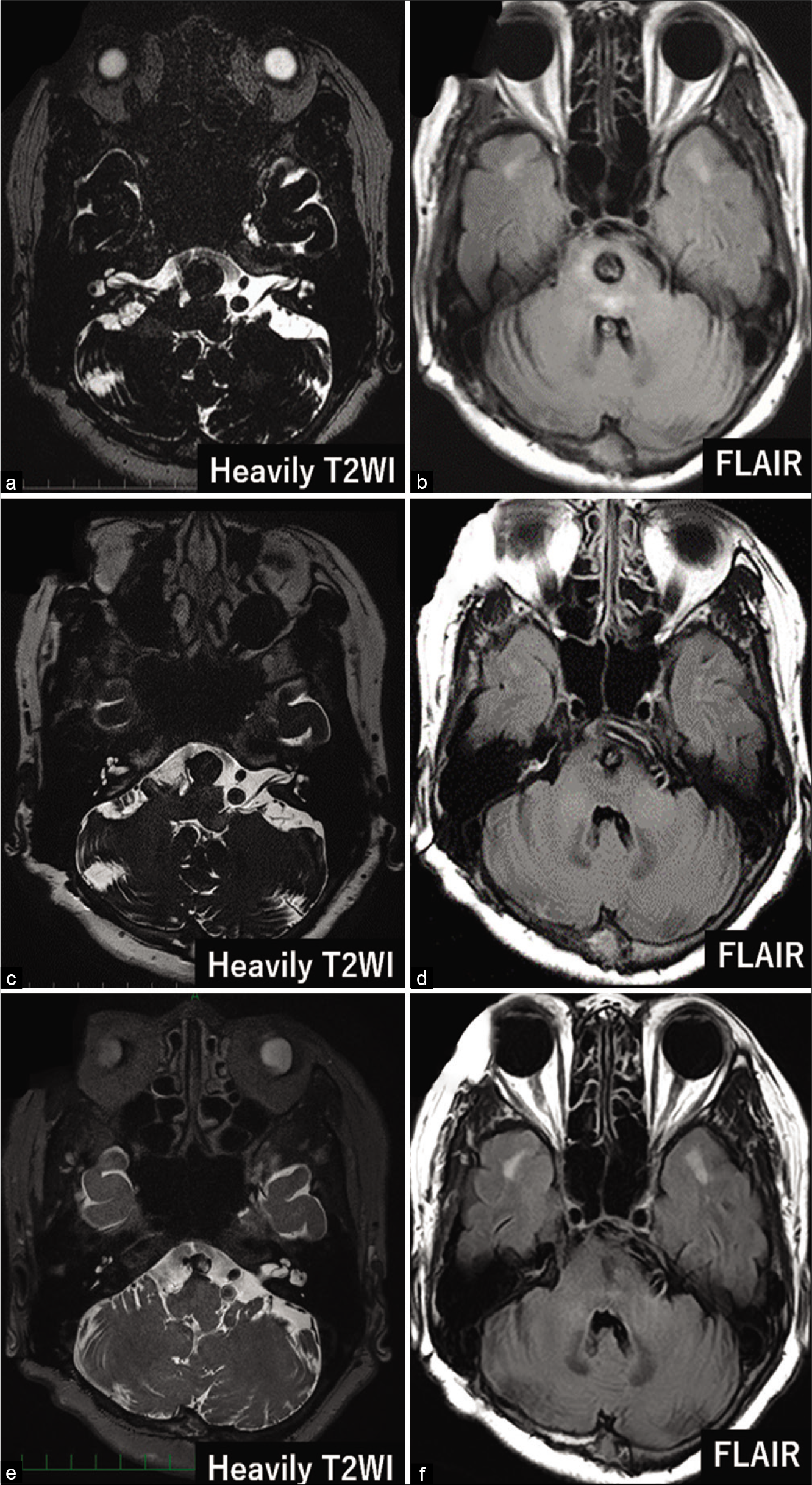
Figure 4:
Imaging findings long after the treatment. (a) Magnetic resonance (MR) image at 6 months postoperatively showing increased aneurysm size (15 mm), (b) MR image at 12 months postoperatively showing decreased aneurysm size (12 mm), (c) MR image at 33 months postoperatively showing further decreased aneurysm size (10 mm), (d) MR image at 6 months postoperatively showing persisting brainstem mass effect, (e) MR image at 12 months postoperatively showing no brainstem mass effect, (f) MR image at 33 months postoperatively showing no brainstem mass effect.
DISCUSSION
No established therapeutic approach for AICA aneurysms exists. Therefore, we have summarized existing literature on proximal AICA aneurysm treatment. Lv et al. found that all premeatal AICA aneurysms were surgically treated by trapping.[
Our patient had an unruptured, partially thrombosed, large, proximal AICA aneurysm. We were concerned about the intolerance to AICA occlusion and planned OA-AICA bypass. Since our patient was asymptomatic, we chose distal clipping, a less invasive treatment, aiming to stop growth by complete aneurysm thrombosis. This might be the second reported case of a proximal AICA aneurysm treated with distal clipping and bypass. Our report is a rare one that describes the postoperative long-term clinical course in detail. If the patient’s condition worsened, we would have considered secondary treatment by parent artery occlusion or trapping; fortunately, there was good progress without these treatments. We identified three reasons for the decreased aneurysm size. First, we could protect the very small arterial branches, which are difficult to visualize through angiography, during microscopic surgery. Actually, we could detect a small branch from the AICA and preserved the branch by just distal clipping of the aneurysm. Second, the vasa vasorum was treated by occluding all vessel wall layers during clipping. Third, intra-aneurysmal flow reduction promotes aneurysm thrombosis and shrinkage.[
Although the availability of new endovascular devices is an important advance, traditional microsurgical techniques can be applied to provide patients with new, minimally invasive treatment strategies.
CONCLUSION
We report a large, partially thrombosed AICA aneurysm. The aneurysm size decreased after distal clipping of aneurysm with OA-AICA bypass. We hope this report will guide clinicians in the treatment of these rare aneurysms.
Ethics approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Drake CG. Giant intracranial aneurysms: Experience with surgical treatment in 174 patients. Clin Neurosurg. 1979. 26: 12-95
2. Fujimura M, Inoue T, Shimizu H, Tominaga T. Occipital artery-anterior inferior cerebellar artery bypass with microsurgical trapping for exclusively intra-meatal anterior inferior cerebellar artery aneurysm manifesting as subarachnoid hemorrhage. Case report. Neurol Med Chir (Tokyo). 2012. 52: 435-8
3. Gonzalez LF, Alexander MJ, McDougall CG, Spetzler RF. Anteroinferior cerebellar artery aneurysms: Surgical approaches and outcomes--a review of 34 cases. Neurosurgery. 2004. 55: 1025-35
4. Halbach VV, Higashida RT, Dowd CF, Barnwell SL, Fraser KW, Smith TP. The efficacy of endosaccular aneurysm occlusion in alleviating neurological deficits produced by mass effect. J Neurosurg. 1994. 80: 659-66
5. Hoh BL, Putman CM, Budzik RF, Carter BS, Ogilvy CS. Combined surgical and endovascular techniques of flow alteration to treat fusiform and complex wide-necked intracranial aneurysms that are unsuitable for clipping or coil embolization. J Neurosurg. 2001. 95: 24-35
6. Hou K, Li G, Xu B, Xu K, Yu J. Which patients with aneurysms involving the a1-a2 segment of the anterior inferior cerebellar artery would benefit from parent artery occlusion?. World Neurosurg. 2019. 126: 301-9
7. Iihara K, Murao K, Sakai N, Soeda A, Ishibashi-Ueda H, Yutani C. Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: The role of vasa vasorum. Case report. J Neurosurg. 2003. 98: 407-13
8. Iwai T, Naito I, Shimaguchi H, Suzuki T, Tomizawa S. Angiographic findings and clinical significance of the anterior and posterior spinal arteries in therapeutic parent artery occlusion for vertebral artery aneurysms. Interv Neuroradiol. 2000. 6: 299-309
9. Lv X, Ge H, He H, Jiang C, Li Y. Anterior inferior cerebellar artery aneurysms: Segments and results of surgical and endovascular managements. Interv Neuroradiol. 2016. 22: 643-8
10. Suh SH, Kim DJ, Kim DI, Kim BM, Chung TS, Hong CK. Management of anterior inferior cerebellar artery aneurysms: Endovascular treatment and clinical outcome. Am J Neuroradiol. 2011. 32: 159-64
11. Tokimura H, Ishigami T, Yamahata H, Yonezawa H, Yokoyama S, Haruzono A. Clinical presentation and treatment of distal anterior inferior cerebellar artery aneurysms. Neurosurg Rev. 2012. 35: 497-503