- Department of Neurosurgery, Instituto Nacional de Ciencias Neurológicas, Lima, Peru,
- Department of Surgical Neuro-Oncology, La Cardio, Bogota, Colombia,
- Department of Neurosurgery, Instituto Nacional de Neurología y Neurocirugía, Mexico City, Mexico.
Correspondence Address:
Rocio Mamani, Department of Neurosurgery, Instituto Nacional de Ciencias Neurológicas, Lima, Perú.
DOI:10.25259/SNI_363_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rocio Mamani1, Javier A. Jacobo2, Gerardo Yoshiaki Guinto-Nishimura3, Alan Hernández-Hernández3, Sergio Moreno-Jimenez3. Motor outcome after resective surgery for the central lobe gliomas. 29-Jul-2022;13:325
How to cite this URL: Rocio Mamani1, Javier A. Jacobo2, Gerardo Yoshiaki Guinto-Nishimura3, Alan Hernández-Hernández3, Sergio Moreno-Jimenez3. Motor outcome after resective surgery for the central lobe gliomas. 29-Jul-2022;13:325. Available from: https://surgicalneurologyint.com/surgicalint-articles/11753/
Abstract
Background: Extent of resection (EOR) plays a major role in the prognosis on patients with gliomas, although the postoperative functionality of the patient is of great importance as well. It is why many surgeons advocate to not operate extensively on tumors that involve eloquent regions such as the central lobe. Recent series have demonstrated that it is possible to achieve extensive resections in this area without significantly affecting the functional outcome for these patients. We illustrate this issue with the experience obtained at the National Institute of Neurology and Neurosurgery in Mexico City.
Methods: This is an observational and retrospective study that included patients that received surgical resection for intracranial gliomas that involved the central lobe at the National Institute of Neurology and Neurosurgery of Mexico, between January 2017 and May 2020. Demographic and clinical variables of the patients at the time of diagnosis were collected as well as tumor morphological variables, surgical adjuncts, and clinical outcomes. Statistical analysis was performed with SPSS software.
Results: A total of 28 patients were included in the study with 43% of patients having a motor deficit before surgery. The average EOR was 88.6%. Patients presented with worsening of their motor status in the immediate postoperative period in 21% of the cases, although most of the patients recovered within the 1st month of follow-up. After analyzing all variables, not having a presurgical motor deficit was a statistically significant risk factor for developing a new motor deficit in the immediate postoperative period (P: 0.02).
Conclusion: A resective surgery for gliomas near or within the central lobe can be performed safely and a satisfactory motor outcome for patients can be achieved without sacrificing the EOR. An intact presurgical motor status is a risk factor for developing a new deficit after surgery.
Keywords: Brain mapping, Central lobe, Glioma, Surgery, Outcome
INTRODUCTION
Glial tumors comprise a heterogeneous pathology, but independently of the histological grade or molecular classification, the extent of resection (EOR) plays an important role in the prognosis of these patients.[
Because of this principle, many surgeons believe that tumors involving so-called eloquent regions are unresectable; one of these areas is the central lobe, which harbors the primary motor and sensory cortex[
In this paper, we show the experience of our institution with the resection of glial tumors within or adjacent to the central lobe.
MATERIALS AND METHODS
This is an observational and retrospective study that included patients that received surgical resection for supratentorial gliomas that involved the central lobe at the National Institute of Neurology and Neurosurgery of Mexico, between January 2017 and May 2020.
Patients over 18 years of age that was taken into surgery for the resection of a glial tumor adjacent or within the central lobe were included in the study, the preoperative clinical motor status was evaluated using the MRC scale, emphasizing whether or not they presented preoperative and postoperative motor or sensory deficits (immediate, 1 month, 3 months, and 6 months after surgery). Other variables that were analyzed are the histological diagnosis and the pre- and post-operative tumor volumes that were measured in cm3 on a presurgical and a 72 h postoperative magnetic resonance imaging (MRI) using the Brainlab SmartBrush software.
Tumor grade was determined using the neuropathology report of our institution, since no molecular diagnosis could be performed due to lack of resources in the majority of cases; we decided to divide gliomas into low- and high-grade pathologies.
For high-grade gliomas, the volume measured was the T1 contrast enhancement region in the preoperative MRI, which was the objective of resection. For low-grade gliomas, the volume measured was the region of abnormal signal intensity in the T2-weighted image, which was the objective of the resection for these tumors, using these measurements, the pre- and post-operative volumes were calculated, the extension of the resection is calculated with the following formula: (100-[vol post/vol pre*100]) and we established that a total gross total resection (GTR) was cutoff at 95% of EOR [
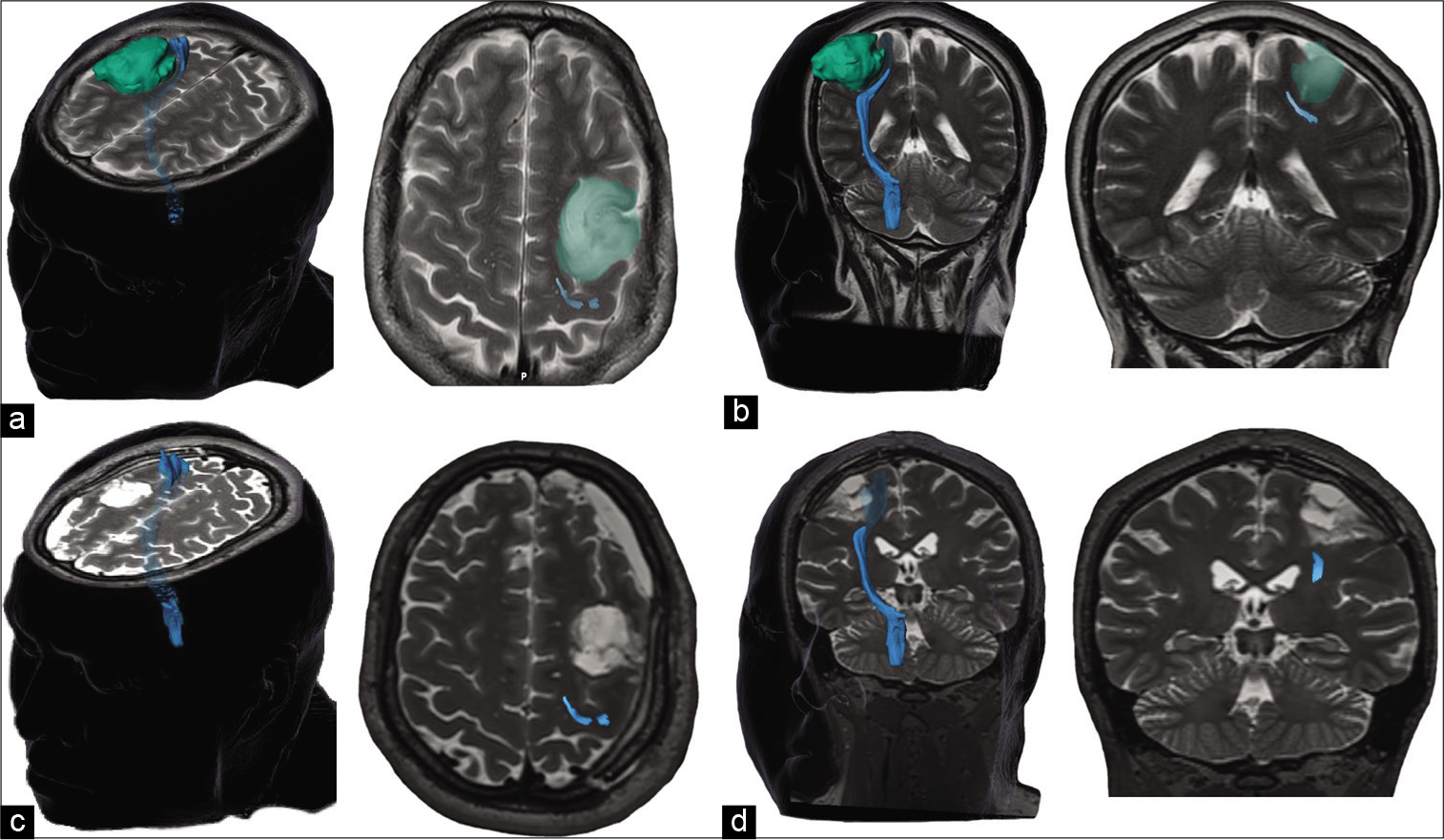
Figure 2:
T2-weighted preoperative MRI in the axial (a) and coronal (b) plane depicting a low-grade glioma in the pre-motor strip (green) that is displacing the pyramidal tract (blue) posteriorly. In the postoperative images (c and d), a gross total resection is seen and the pyramidal tract is back to its usual anatomical location.
The Karnofsky performance status (KPS) was also evaluated before and after surgery, as well as early complications typical of surgery, except for motor deficit, which were evaluated separately.
Patients requiring a reintervention and with tumors well beyond the central lobe were excluded from the study.
Perioperative management
Preoperative management: All patients who underwent awake craniotomy had a presurgical neuropsychological evaluation and those who received anticonvulsants received their usual dose before surgery. Intraoperative management:
Anesthesia protocol: Patients were subjected to two types of anesthesia (the asleep-awake-asleep protocol or general anesthesia) according to the protocols already established in our institution, the type of anesthesia depended on the previous assessment of the group as a whole (neurosurgery, neuropsychology, and neuroanesthesiology) Muscle relaxants were avoided and used only during endotracheal intubation as appropriate Surgical technique: The patient’s head is fixed with a Mayfield fixation system, in which the support of the neuronavigation system was mounted when it was used to plan the craniotomy. The craniotomy was centered and tailored according to the size and location of the tumor Brain mapping: Immediately before tumor resection, the neuronavigation system is used to visualize the functional areas as guided by the DTI tractography and their relationship with the tumor; cortical electric stimulation was carried out either with the patient fully awake or under general anesthesia, depending on whether only motor pathways were planned to be stimulated or also regions related to speech and language The Penfield technique was used using the Ojemann stimulator (Integra OCS2), with a separation of 5 mm between the electrodes, the Ojemann stimulator produces a constant current that produces a train of square wave, biphasic pulses of phase duration from 1 millisecond in a frequency of 60 Hz. For the location of an eloquent area, the intensity of the electric stimulus was typically started at 1 mA and increased in a stepwise manner by 1 mA, a cortical area was considered eloquent if a motor response or paresthesia was generated, or if speech errors were made consistently in at least three separate trials and then a number or letter were placed on the stimulated cortical area No cortical area was stimulated twice in a row. As a general rule, 4–6 mA was the maximum stimulus necessary to locate the functional area in awake patients, if there was no response when reaching 8 mA, the area was considered “silent,” no further increase in amperage was made, as it considerably increases the risk of intraoperative seizures. In patients under general anesthesia, the current was taken up to 10 mA If clinically evident intraoperative seizures occurred, the electric stimulation was stopped and the cerebral cortex was irrigated with cold 0.9% saline solution and was repeated as necessary. If this seizure lasted more than 120 s, 30–40 mg boluses of propofol were administered, taking care not to cause respiratory distress in the awake patient, also intravenous phenytoin or levetiracetam was added in a dose equivalent to the current intake of the patient or impregnation doses were used if the patient did not previously take anticonvulsants. If the seizure persisted despite this treatment for more than 5 min, propofol infusion was initiated to control the seizure and orotracheal intubation was performed. Tumor resection: After locating the eloquent areas, the tumoral resection was carried out until reaching the positive cortical or subcortical mapping sites
A microsurgical technique was used during the resection and emphasis was placed on respecting the arachnoid plane and its respective blood vessels that surrounded the functional areas, care was taken to manipulate said area as little as possible and if hemostasis is required, we used hemostats such as Surgicel to avoid using the bipolar coagulator as much as possible. The venous drainage of the resected region was protected using skeletonizing techniques and sacrificing veins as little as possible [
Figure 3:
Intraoperative image before tumor resection (a) that depicts the macroscopic periphery of the tumor (green line) and the positive motor sites as it was found according to the motor cortex stimulation (paper squares 1 and 2). After tumor resection (b), a large cavity is visible and the eloquent regions as well as the venous drainage are spared.
Statistical analysis
Demographic and clinical variables of the patients at the time of diagnosis were collected, including sex, age, clinical manifestations, and initial functional status as assessed by the KPS scale. Tumor morphological variables were collected, including affected side, location in relation to the central lobe, and volume. Surgical adjuncts such as neuronavigation, awake craniotomy, and intraoperative cortical stimulation were recorded, as well as intraoperative findings such as seizures and complications. Clinical outcome was recorded until last follow-up visit and included mortality, muscular strength, and functional status as assessed by KPS. Radiological outcome was assessed by EOR with volumetric analysis, with GTR defined as >95% resection.
Statistical analysis was performed with SPSS software (version 23.0; IBM Corp., Armonk, NY). We performed a descriptive analysis, reporting frequencies of categorical variables, as well as mean and standard deviation of continuous variables. A Wilcoxon signed-rank test was used to compare muscular strength before and after surgery using different time points in follow-up (immediately after surgery and at 1-, 3-, and 6-month follow-up). A comparative analysis of the collected variables was performed between patients who presented with transient postoperative muscular strength worsening and patients who remained stable after surgery. We used Student’s t-test for normally distributed and Mann–Whitney U-test for nonparametric continuous variables, and Chi-squared test for categorical variables. P < 0.05 was considered statistically significant.
RESULTS
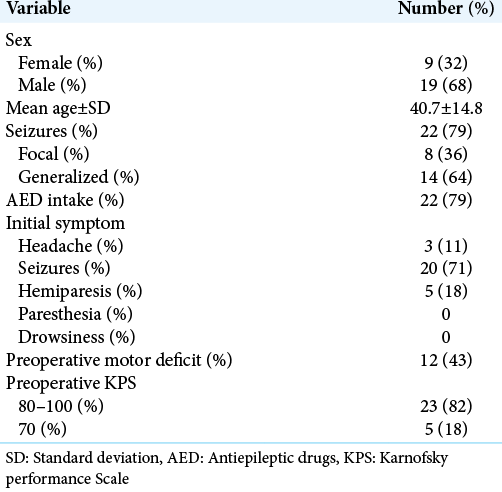
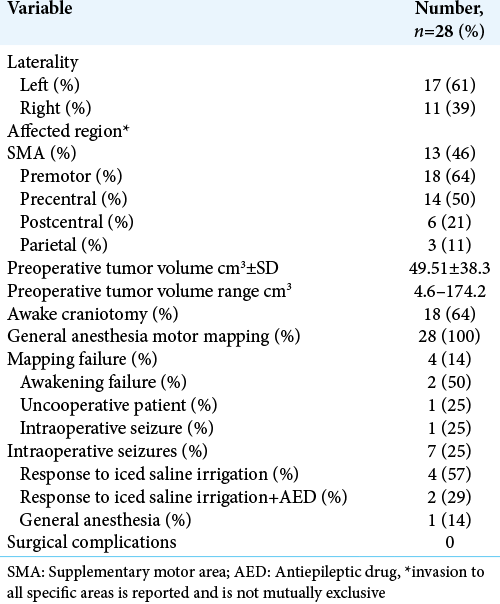
A total of 28 patients were included in the study, of which 68% were male patients. The average age for our study group was 40 years, and 23 out of the 28 patients had a KPS above 80%. About 43% of patients had a motor deficit before surgery.
Out of the 28 patients, 71% had tumors that involved either in the precentral or postcentral gyrus, the rest of the tumors involved the supplementary motor cortex, the premotor region, or the parietal lobe. The preoperative tumor volume was 49 cubic centimeters on average. Direct cortical motor mapping was performed in every single case to identify and localize the pyramidal tract, even in those surgeries where the patient was eventually awakened to performed additional motor and speech tasks.
The average EOR was 88.6% while 39% of cases received a GTR, defined as an EOR superior to 95%.
There was no significant difference between the EOR for HGG and LGG. The average EOR in HGG was 86.6% and for LGG was 89.7%.
In the MRC motor scale, 82% of patients had a motor strength of 4–5, that number dropped to 61% in the immediate postoperative period. After a 6-month follow-up, 79% of patients scored 4 or 5 in the motor scale, and they recovered their motor functionality within the 1st month of follow-up period [
Figure 4:
Motor strength. Stacked bar graph showing the patients’ strength throughout follow-up. The proportion of patients within each grade according to MRC score is compared before, immediately after, and at 1, 3, and 6 months after surgery. Baseline motor strength was significantly different to immediate postoperative strength (P = 0.015), but similar to long-term motor strength (P = 0.82).
After analyzing all variables, not having a presurgical motor deficit was a statistically significant risk factor for developing a new motor deficit in the immediate postoperative period (P: 0.02).
DISCUSSION
The central lobe is a complex region within the brain, which harbors some of the most eloquent structures for human beings: the motor and sensory pathways. Recent advances in microsurgical techniques, modern neuroimaging, neuronavigation systems, and cortical mapping have made surgery for tumors in the central lobe safer.[
The goal of this retrospective analysis was to describe the temporary or permanent motor deficit that patients develop postoperatively after resective surgery for tumors within or adjacent to the central lobe, and the feasibility of performing this surgery using different techniques for motor mapping.
We present a series of 28 patients that underwent surgery for the resection of a glioma in close relationship to the central lobe and shown that nearly 73% of patients will develop a transient worsening in the motor strength scale in the immediate postoperative period, but nearly all of the patients will recover within the first 3 months after surgery.
One of the first series that addressed the issue of motor outcome after surgery for gliomas in close relationship to the motor pathways was done by Duffau et al.[
In the largest documented series, the authors from two neurosurgical centers described their experience in resecting gliomas located within or adjacent to the descending motor pathways using intraoperative subcortical stimulation mapping in 294 patients.[
In our study, the only factor that we could identify that relates to the risk of developing a new motor deficit was the presurgical motor scale; patients who before surgery had no motor deficit were more likely to develop a new deficit in the immediate postoperative period. We believe that this may be explained because a new motor deficit may be more apparent in a patient that was previously intact, rather than a patient with a prior deficit; also, a more robust functional bundle of fibers may be more easily damaged than a motor tract that had less functional fibers to begin with.
Other factors such as the tumor location, tumor volume, and EOR did not have a significant impact on the motor outcome of these patients.
In our series, we used DTI tractography as a way to plan the approach for the surgery, but not as a predictive method. In a recent study, Cepeda and colleagues showed that the preoperative fractional anisotropy in the peritumoral edema could be used to predict permanent deficit after surgical resection of gliomas adjacent to the corticospinal tract.[
DTI tractography has been used recently in the intraoperative setting using augmented reality. Superimposed images in real time of the reconstructed tracts can be visualized using modern microscopes and aid in the sparing of eloquent tracts and appear to be helpful to achieve greater EOR without sacrificing functional outcomes.[
More recently the group of the University of California, San Francisco published their experience with surgery for tumors located primarily within the motor cortex and concluded that an excellent EOR can be achieved without negatively impacting the outcome of these patients, suggesting that these tumors are indeed amenable to resection and should not be labeled as unresectable.[
In their series, they included 49 patients with gliomas within the motor cortex that went into surgery for resection. Out of the 49 patients, 60% developed new motor deficit and 20% developed a permanent new deficit, although only 4% were considered severe. It is interesting that in this study, an awake craniotomy was performed in only 65% of cases, and this did not affect either the EOR or the motor outcome for the patients.
In our surgical series, 64% of surgeries were performed with an awake cortical stimulation technique and found that the motor outcome did not relate to the technique used for motor mapping. These results are consistent with a recent meta-analysis that concluded that the resection of gliomas located in or near the perirolandic area and descending motor tracts can be safely carried out with both awake craniotomy and general anesthesia.[
According to our results, the EOR for gliomas near the motor tract does not have a relevant impact with the final motor outcome for these patients, as it was established in the previous series.[
It is important to make emphasis that several situations may alter the surgeon’s ability to perform an adequate mapping and achieve an optimal EOR with the best neurological results. Such situation may be intraoperative seizures, which in our series were the reason for mapping failure in 25% of the cases, without any significant impact on the final motor status of the patients. In a recent paper published by our group, the only risk factor associated with the presence of intraoperative seizures was tumor volume, as larger tumors were more likely to develop seizures during brain mapping. Other factors such as tumor location or tumor histology were not identified as risk factors.[
CONCLUSION
A resective surgery for gliomas near or within the central lobe can be performed safely and a satisfactory motor outcome for patients can be achieved without sacrificing the EOR, although every case should be individualized and maximal safe resection should always be the ultimate goal in eloquent region located gliomas. Patients without a prior motor deficit were at risk for developing a new deficit after surgery.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016. 2: 1460-9
2. Cahill DP. Extent of resection of glioblastoma: A critical evaluation in the molecular era. Neurosurg Clin N Am. 2021. 32: 23-9
3. Cepeda S, García-García S, Arrese I, Velasco-Casares M, Sarabia R. Acute changes in diffusion tensor-derived metrics and its correlation with the motor outcome in gliomas adjacent to the corticospinal tract. Surg Neurol Int. 2021. 12: 51
4. Chambless LB, Kistka HM, Parker SL, Hassam-Malani L, McGirt MJ, Thompson RC. The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neurooncol. 2015. 121: 359-64
5. Duffau H, Capelle L, Sichez J, Faillot T, Abdennour L, Koune JD. Intra-operative direct electrical stimulations of the central nervous system: the Salpêtrière experience with 60 patients. Acta Neurochir (Wien). 1999. 141: 1157-67
6. Duffau H, Denvil D, Capelle L. Absence of movement disorders after surgical resection of glioma invading the right striatum. J Neurosurg. 2002. 97: 363-9
7. Eisner W, Burtscher J, Bale R, Sweeney R, Koppelstätter F, Golaszewski S. Use of neuronavigation and electrophysiology in surgery of subcortically located lesions in the sensorimotor strip. J Neurol Neurosurg Psychiatry. 2002. 72: 378-81
8. Frigeri T, Paglioli E, de Oliveira E, Rhoton AL. Microsurgical anatomy of the central lobe. J Neurosurg. 2015. 122: 483-98
9. Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: Evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004. 100: 369-75
10. Luzzi S, Lucifero AG, Martinelli A, Del Maestro M, Savioli G, Simoncelli A. Supratentorial high-grade gliomas: maximal safe anatomical resection guided by augmented reality high-definition fiber tractography and fluorescein. Neurosurg Focus. 2021. 51: E5
11. Magill ST, Han SJ, Li J, Berger MS. Resection of primary motor cortex tumors: Feasibility and surgical outcomes. J Neurosurg. 2018. 129: 961-72
12. Mamani R, Jacobo JA, Mejia S, Nuñez-Velasco S, AragonArreola J, Moreno S. Analysis of intraoperative seizures during bipolar brain mapping in eloquent areas: Intraoperative seizures in brain mapping. Clin Neurol Neurosurg. 2020. 199: 106304
13. Suarez-Meade P, Marenco-Hillembrand L, Prevatt C, MurguiaFuentes R, Mohamed A, Alsaeed T. Awake vs asleep motor mapping for glioma resection: A systematic review and meta-analysis. Acta Neurochir (Wien). 2020. 162: 1709-20
14. Sun GC, Wang F, Chen XL, Yu XG, Ma XD, Zhou DB. Impact of virtual and augmented reality based on intraoperative magnetic resonance imaging and functional neuronavigation in glioma surgery involving eloquent areas. World Neurosurg. 2016. 96: 375-82