- Department of Neurosurgical Oncology, Mexico City, Mexico
- Department of Radio-Neurosurgery, Mexico City, Mexico
- Department of Neurosurgery, National Institute of Neurology and Neurosurgery “Manuel Velasco Suarez”, Mexico City, Mexico
- Department of Neurosurgery, University of La Salle, Mexico City, Mexico.
Correspondence Address:
Sonia Iliana Mejia-Perez, Department of Neurosurgical Oncology, National Institute of Neurology and Neurosurgery, Mexico City, Mexico.
DOI:10.25259/SNI_104_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jose Omar Santellan-Hernandez1, José Alfonso Alvarez-Castro1, Keren Magaly Aguilar-Hidalgo1, Fernando Castro Soto2, Jonathan Ramos Escalante3, Eduardo Ichikawa-Escamilla3, Maria Jose Alvarez Silva4, Sonia Iliana Mejia-Perez1. Multifocal glioblastoma and hormone replacement therapy in a transgender female. 24-Mar-2023;14:106
How to cite this URL: Jose Omar Santellan-Hernandez1, José Alfonso Alvarez-Castro1, Keren Magaly Aguilar-Hidalgo1, Fernando Castro Soto2, Jonathan Ramos Escalante3, Eduardo Ichikawa-Escamilla3, Maria Jose Alvarez Silva4, Sonia Iliana Mejia-Perez1. Multifocal glioblastoma and hormone replacement therapy in a transgender female. 24-Mar-2023;14:106. Available from: https://surgicalneurologyint.com/surgicalint-articles/12207/
Abstract
Background: Glioblastoma multiforme represents approximately 60% of all brain tumors in adults. This malignancy shows a high level of biological and genetic heterogeneity associated with exceptional aggressiveness, leading to poor patient survival. One of the less common presentations is the appearance of primary multifocal lesions, which are linked with a worse prognosis. Among the multiple triggering factors in glioma progression, the administration of sex steroids and their analogs has been studied, but their role remains unclear to date.
Case Description: A 43-year-old transgender woman who has a personal pathological history of receiving intramuscular (IM) hormone treatment for 27 years based on algestone/estradiol 150 mg/10 mg/mL. Three months ago, the patient suddenly experienced hemiplegia and hemiparesis in her right lower extremity, followed by a myoclonic focal epileptic seizure, vertigo, and a right frontal headache with a visual analog scale of 10/10. Magnetic resonance imaging images revealed an intra-axial mass with poorly defined, heterogeneous borders, and thick borders with perilesional edema in the left parietal lobe, as well as a rounded hypodense image with well-defined walls in the right internal capsule. The tumor was resected, and samples were sent to the pathology department, which confirmed the diagnosis of wild-type glioblastoma.
Conclusion: This report identifies prolonged use of steroid-based hormone replacement therapy as the only predisposing factor in the oncogenesis of multifocal glioblastoma. It is an example that highlights the importance for physicians not to consider pathologies related to the human immunodeficiency virus rather than neoplasms in transgender patients in view of progressive neurological deterioration.
Keywords: Gender, Glioblastoma, Multicentric, Neurosurgery, Transgender
INTRODUCTION
Glioblastoma is a highly malignant type of tumor that originates from neuroglial cells in the central nervous system. It is commonly seen in people aged between 45 and 70 years old[
According to the United Nations, transgender people have a gender identity different from the sex that they were assigned at birth. This gender identity is distinct from sexual orientation, as the former refers to how someone identifies themselves, and the latter refers to emotional and sexual attraction to another person of the same or different sex. To affirm their gender identity, trans people often undergo surgeries and hormone treatments, commonly with estrogen and cyproterone acetate.[
Cancer progression after cross-sex therapy has been reported in different tissues. Mortality rates for lung and hematological malignancies have been identified as being increased after hormone replacement therapy (HRT); moreover, there are estrogen-sensitive breast carcinomas and a few cases of prostate carcinomas reported in transwomen who started cross-sex therapy after the age of 50.[
Regarding glioblastomas, there are cases of pregnant women between 20 and 40 years old, where progesterone levels reach serum levels up to 200-fold higher than baseline; they presented the first clinical manifestation or acceleration of symptoms due to brain tumors, among which 55 of 119 cases were gliomas (31% with estrogen receptors and 31% with progesterone receptors) with a poor postpartum amelioration.[
Given that cross-hormonal therapy is an ongoing therapy for these patients, vigilance for possible side effects such as those mentioned above is essential. However, since there is no specific screening for the early identification of brain tumors, including glioblastomas, it is possible that there is an underestimation of the actual prevalence of these tumors in transgender patients receiving such treatment.
CASE PRESENTATION
A 43-year-old, right-handed transgender woman from the State of Mexico is a housewife and professes the Catholic religion. Three months ago, the patient suddenly experienced hemiplegia and hemiparesis in her right lower extremity, followed by a myoclonic focal epileptic seizure, vertigo, and a right frontal headache with a visual analog scale of 10/10. In the past month, the patient reported drowsiness, urinary sphincter loss, diplopia for 1 week, three daily focal epileptic seizures in the right arm and leg with tonic-clonic characteristics, without alterations of the mental status, and postictal weakness.
The patient has a personal pathological history of receiving intramuscular (IM) hormone treatment for 27 years based on algestone/estradiol 150 mg/10 mg/mL, which is currently suspended. She has been vaccinated against COVID on three occasions with the Pfizer brand and has not contracted the disease. In addition, she has undergone several surgical interventions, such as rhinoplasty 25 years ago, breast implants 20 years ago, and other cosmetic surgeries on the face. With a significant family history of illness, her mother has been diagnosed with Parkinson’s disease; her father suffered from diabetes mellitus and died of liver cancer, and her brother has a history of deep vein thrombosis of the lower extremities.
During the clinical examination, right eye exotropia, bradykinesia, anisocoria with the right miosis, right-side spasticity, and left side exteroceptive hypoesthesia were noted. To treat her adjustment disorder with depressive symptoms due to dysplastic hyperthymia, a tendency to cry, and ideas of worthlessness and ruin, she started on mirtazapine at 15 mg daily.
She was admitted to a private hospital and started on carbamazepine 200 mg every 8 h as symptomatic treatment. However, 9 days later, her condition had not improved, so a computerized axial tomography was requested. This showed encephalic volume with structural changes according to age and a lesion of primary neoplastic aspect, likely a glioblastoma, in the left parietal lobe subcortical towards the convexity.
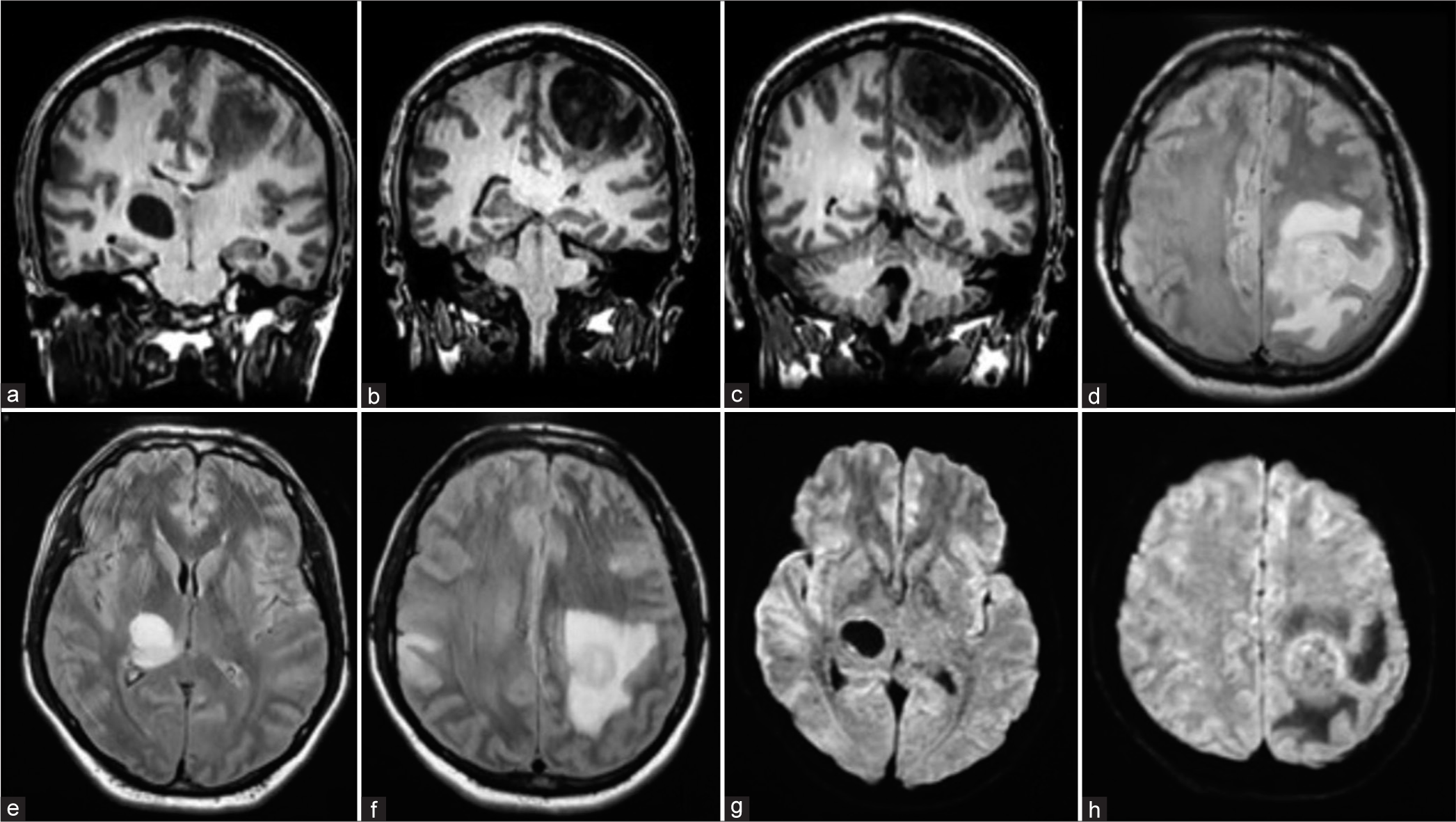
On arrival at our institution, magnetic resonance imaging image studies were performed [
After establishing the diagnosis and determining the surgical strategy, the patient was transferred to the operating room under anesthesia and placed in a supine decubitus position with Mayfield-Kees cranial fixation. A craniotomy-guided horseshoe incision centered on the lesion was made, with intraoperative ultrasonographic tracing to confirm the lesion below the craniotomy. A lesion was identified from the subcortical region with a soft, friable consistency, and yellowish appearance, measuring approximately 3.5 × 4.5 cm in diameter. Due to its friability, fragmentary resection was performed, with the lesion being devastated using Mallis forceps and suction. Samples from the resected tumor were sent to the pathology department, which confirmed the diagnosis of wild-type glioblastoma [
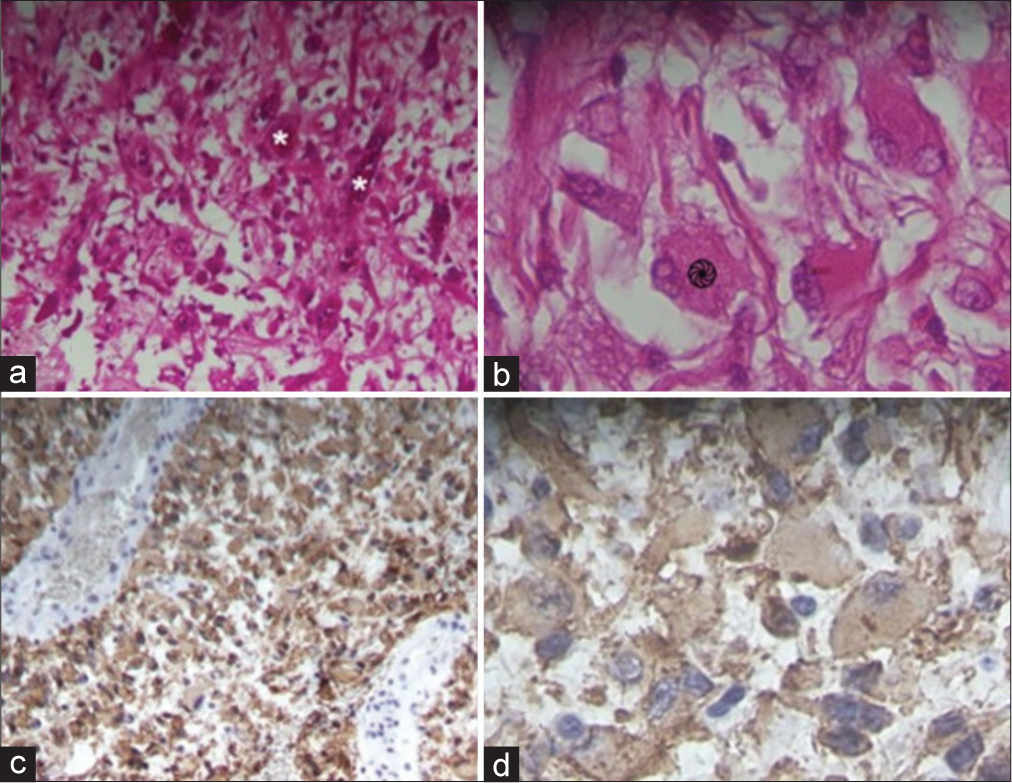
Figure 2:

Histopathological findings of the resected tumor. A left frontoparietal lesion with a grayish-brown, hemorrhagic appearance is observed. (a and b) Hematoxylin and eosin staining reveal giant cells (* -
The patient was discharged from the operating room in a hemodynamically stable state and with a functioning ventilator. Postoperative imaging studies were conducted [
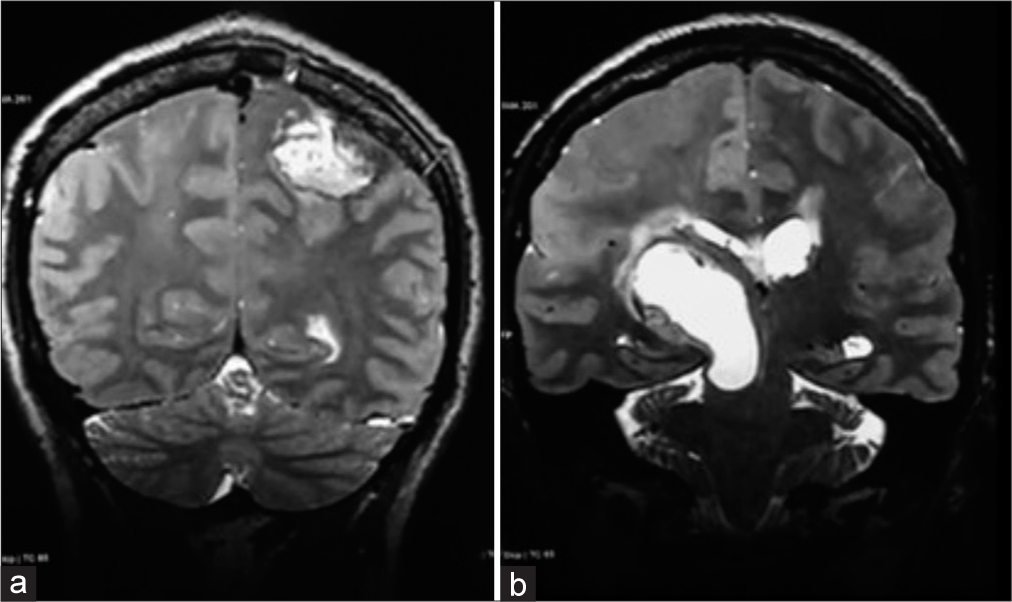
Figure 3:
Skull magnetic resonance imaging (MRI). December 15, 2022. Postoperative. (a and b) Coronal axis T2 MRI sequence. Post-surgical changes are observed in the left frontotemporal region, with a well-defined post-surgical space present in 90% of resections. In addition, the right thalamic lesion is larger in size and is compressing the lateral ventricle of the same hemisphere.
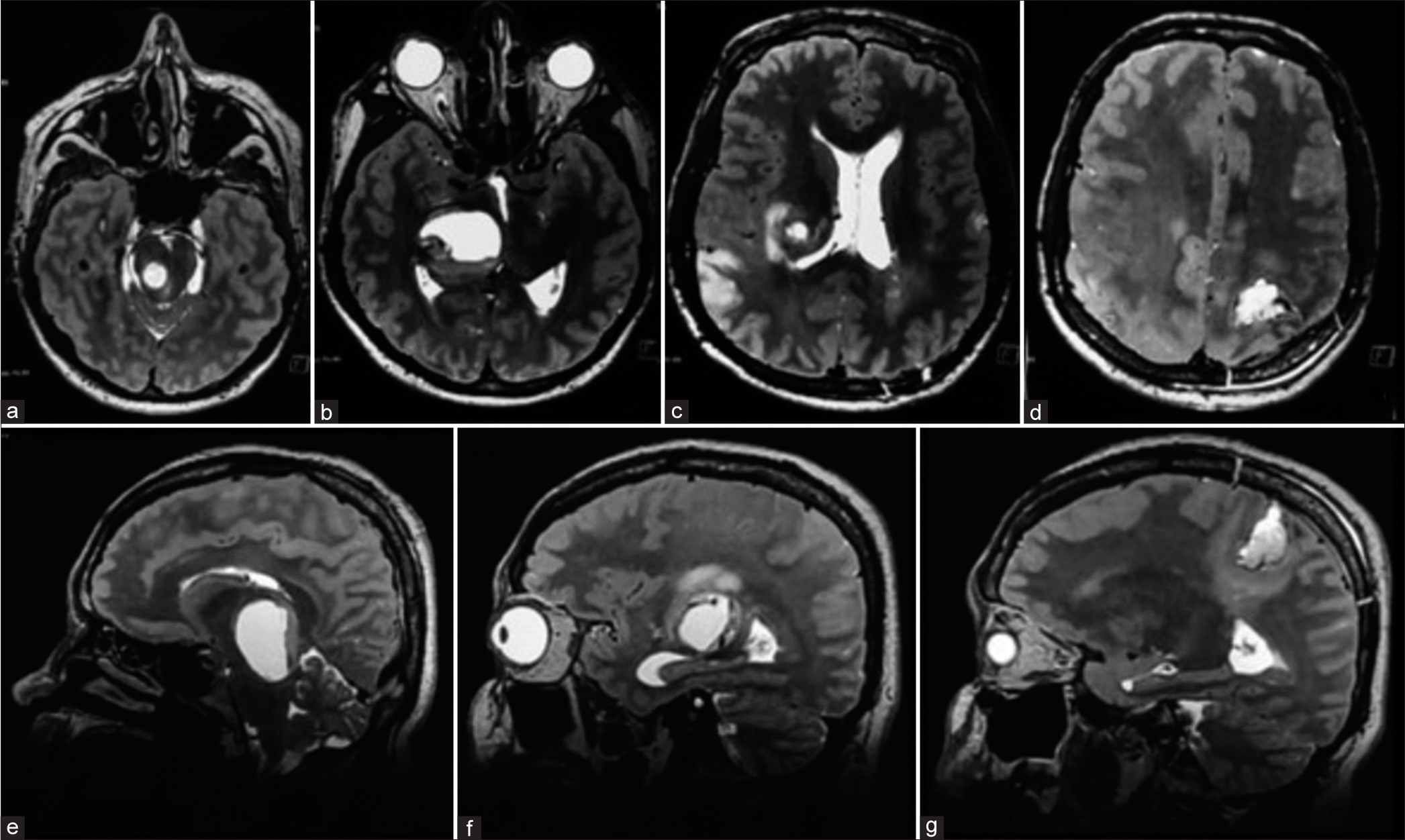
Figure 4:
Skull magnetic resonance imaging (MRI), January 09, 2023, postoperative. Axial axis T2 MRI sequence (a-d) and sagittal axis T2 MRI sequence (e-g) show a right thalamic lesion beginning to invade the pons from the midbrain and extending to the corpus callosum, with perilesional edema covering much of the frontal lobe. In addition, a larger area of hyperintensity is observed in the right parietal cortical lesion. The left frontoparietal post-surgical space is well-demarcated.
DISCUSSION
Although there are no similar cases reported of multifocal glioblastomas in transgender people, it is essential to acknowledge that transgender people represent a heterogeneous population group and that cancer risks, hormone use, and the prevalence of non-hormonal risk factors may vary widely across the gender identity spectrum.[
The patient, in this case, used algestone and estradiol as dual HRT for a prolonged period. Therefore, it is essential to emphasize the role of sex hormones such as androgens, estrogens, and progestogens as possible factors in the development and progression of glioblastoma. Otherwise, there is a historical context that has associated the use of androgens with the incidence of cancers of different histological lineages, the most related being prostate cancer. In addition, there has been an interest in researching treatments that antagonize the effects of this type of hormonal therapy. An example of this is the interest in investigating androgen receptor (AR) positivity and subsequent treatment with androgen antagonists.[
Among the hormonal factors associated with glioblastoma development and progression are estrogens. These hormones activate the G protein-coupled receptor (GPER1), which interacts with hypoxia-inducible factor-1α to promote the expression of genes encoding growth factors such as connective tissue growth factor, vascular endothelial growth factor (VEGF), and interleukin 6, which are related to oncogenesis in different tissues. The regulatory role of GPER1 in glioma progression could be a promising area of research. On the other hand, estrogen receptor alpha (ERα) has a low expression in adult neural stem cells, but its isoform ERα36 is expressed in glioblastoma cell lines and is related to tamoxifen resistance.[
Finally, progestins are another type of sex hormone thought to be associated with glioblastoma. Specifically, it has been reported that they can increase glial tumor cell proliferation at low doses, although they also induce cell differentiation and apoptosis at higher doses. In murine models, low-dose administration of progesterone (10 nM) significantly increased glioblastoma tumor area and infiltration length. This hormone induces that the synthesis of cyclin D1, epidermal growth factor receptor, progesterone-induced blocking factor, and steroid receptor coactivator-1, is involved in the induction of VEGF in human glioma cells, and activates cofilin, a protein that promotes the formation of free barbed ends and tumor invasion.[
Despite mentioning several variables that may be associated with the development of gliomas, the only established risk factors are high doses of ionizing radiation and certain rare genetic conditions. However, the use of exogenous hormones may also have a potential role in the etiology. Studies such as that of Gonzalez et al. (2021) reaffirm the importance of the hormonal context in glioblastoma.[
CONCLUSION
Glioblastoma is a high-grade tumor that occurs in both male and female patients. In this case of a transgender female who has undergone HRT, there may be a potential risk of developing multiple glioblastomas due to the increased levels of hormones that can stimulate the growth of cancer cells. Although several studies suggest the use of progestins and their analogs as protective factors in brain tumors, this case leans more toward the role of estrogens and progestins in tumor progression. However, further research is needed to confirm this conclusion.
From an ethical standpoint, physicians must have an objective assessment when diagnosing patients with a transgender role, since it is common for physicians to orient toward pathologies related to the human immunodeficiency virus rather than neoplasms. This case exemplifies the appearance of a primary tumor of the central nervous system without bias in the diagnosis after understanding the patient’s clinical history.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
This paper and the research behind it would not have been possible without the exceptional support, of Dr. Sonia Mejía, and Dr. Omar Santellán, as well as all the colleagues who helped write this paper, Dr. Alfonso Alvarez, Dr. Magaly Aguilar, Dr. Fernando Castro, Dr. Jonathan Ramos, Dr. Eduardo Ichikawa, and Dr. María José Alvarez.
References
1. Abou-El-Ardat K, Seifert M, Becker K, Eisenreich S, Lehmann M, Hackmann K. Comprehensive molecular characterization of multifocal glioblastoma proves its monoclonal origin and reveals novel insights into clonal evolution and heterogeneity of glioblastomas. Neuro Oncol. 2017. 19: 546-57
2. Altinoz MA, Ozpinar A, Elmaci I. Reproductive epidemiology of glial tumors may reveal novel treatments: High-dose progestins or progesterone antagonists as endocrine-immune modifiers against glioma. Neurosurg Rev. 2019. 42: 351-69
3. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011. 164: 635-42
4. Barnard RO, Geddes JF. The incidence of multifocal cerebral gliomas. A histologic study of large hemisphere sections. Cancer. 1987. 60: 1519-31
5. Benson VS, Pirie K, Green J, Bull D, Casabonne D, Reeves GK. Hormone replacement therapy and incidence of central nervous system tumors in the Million Women Study. Int J Cancer. 2010. 127: 1692-8
6. Boer M, Moernaut L, Van Calenbergh F, Lapauw B, T’Sjoen G. Variation of meningioma in response to cyproterone acetate in a trans woman. Int J Transgend. 2018. 19: 92-4
7. Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M. Cancer in transgender people: Evidence and methodological considerations. Epidemiol Rev. 2017. 39: 93-107
8. Di Carlo DT, Cagnazzo F, Benedetto N, Morganti R, Perrini P. Multiple high-grade gliomas: Epidemiology, management, and outcome. A systematic review and meta-analysis. Neurosurg Rev. 2019. 42: 263-75
9. González-Mora AM, Garcia-Lopez P. Estrogen receptors as molecular targets of endocrine therapy for Glioblastoma. Int J Mol Sci. 2021. 22: 12404
10. Kabat GC, Park Y, Hollenbeck AR, Schatzkin A, Rohan TE. Reproductive factors and exogenous hormone use and risk of adult glioma in women in the NIH-AARP diet and health study. Int J Cancer. 2011. 128: 944-50
11. Khandwala K, Mubarak F, Minhas K. The many faces of glioblastoma: Pictorial review of atypical imaging features. Neuroradiol J. 2021. 34: 33-41
12. Li Y, Zhang ZX, Huang GH, Xiang Y, Yang L, Pei YC. A systematic review of multifocal and multicentric glioblastoma. J Clin Neurosci. 2021. 83: 71-6
13. Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology Practice Guideline. J Clin Oncol. 2007. 25: 1596-605
14. McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD. Complexities of androgen receptor signaling in breast cancer. Endocr Relat Cancer. 2014. 21: T161-81
15. Meriggiola MC, Gava G. Endocrine care of transpeople Part II. A review of cross-sex hormonal treatments, outcomes, and adverse effects in transwomen. Clin Endocrinol (Oxf). 2015. 83: 607-15
16. Naderi A, Chia KM, Liu J. Synergy between inhibitors of androgen receptor and MEK has therapeutic implications in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011. 13: R36
17. Reyes GA. Gliomas del adulto: Acercamiento al diagnóstico y tratamiento. Acta Neurol Colomb. 2009. 25: 34-41
18. Roelvink NC, Kamphorst W, van Alphen HA, Rao BR. Pregnancy-related primary brain and spinal tumors. Arch Neurol. 1987. 44: 209-15
19. United Nations Human Rights Office. Transgender. United Nations Free and Equal. Available from: https://www.unfe.org/wp-content/uploads/2017/05/unfe-transgender.pdf [Last accessed on 2023 Jan 23].
20. Ware KE, Garcia-Blanco MA, Armstrong AJ, Dehm SM. Biologic, and clinical significance of androgen receptor variants in castration-resistant prostate cancer. Endocr Relat Cancer. 2014. 21: T87-103