- Department of Radiology, Aga Khan University Hospital, Karachi, Pakistan,
- Department of Surgery, Aga Khan University Hospital, Karachi, Pakistan,
- Department of Pathology and Laboratory Medicine, Aga Khan University Hospital, Karachi, Pakistan.
Correspondence Address:
Kumail Khandwala, Department of Radiology, Aga Khan University Hospital, Karachi, Pakistan.
DOI:10.25259/SNI_589_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Fatima Mubarak1, Kumail Khandwala1, Shahzad M. Shamim2, Madiha Bilal Qureshi3. Multifocal oligodendroglioma with callosal and brainstem involvement. 30-Sep-2022;13:442
How to cite this URL: Fatima Mubarak1, Kumail Khandwala1, Shahzad M. Shamim2, Madiha Bilal Qureshi3. Multifocal oligodendroglioma with callosal and brainstem involvement. 30-Sep-2022;13:442. Available from: https://surgicalneurologyint.com/surgicalint-articles/11908/
Abstract
Background: Oligodendrogliomas are generally low-grade glial neoplasms commonly occurring in a cortical or subcortical location and frequently contain coarse calcifications. Tumors with 1p and 19q codeletions behave atypically and are more likely to have ill-defined margins and tend to have calcification. Very rarely, diffuse pattern and gliomatosis type of infiltrative nature of oligodendrogliomas have been described in sporadic case reports.
Case Description: In this article, we present a case of a 31-year-old male who had diffuse multifocal oligodendroglioma with rare features of extensive callosal and brainstem involvement on imaging.
Conclusion: Rare cases of oligodendrocytic gliomatosis cerebri or oligodendrogliomatosis with diffuse white matter spread of these tumors usually lead to a detrimental course of neurological status and a poor prognosis in these patients.
Keywords: Brain, Infiltration, Oligodendroglioma, Spectroscopy, Tumor
INTRODUCTION
Oligodendroglioma is glial neoplasms and they represent up to 5–20% of all gliomas.[
CASE PRESENTATION
A 31-year-old male patient presented with a 3-month history of gradually worsening headache and progressive left-sided weakness. He denied any history of seizures or any other significant medical or surgical history. On examination, the patient had papilledema, left-sided upper motor neuron type facial weakness, and left hemiparesis (MRC Grade 4) with pronator drift. Plantars were upgoing on the left side, suggesting complete lateralization. Rest of the examination was unremarkable.
Magnetic resonance imaging (MRI) revealed diffuse signal abnormality, multifocal lesions in the right frontal, temporal lobes with an involvement of the right thalamus, and extension into the right half of the brainstem up to the pons. Some of the lesions were cortical and subcortical in location and cystic changes were also seen in the tumor on T2-weighted imaging [
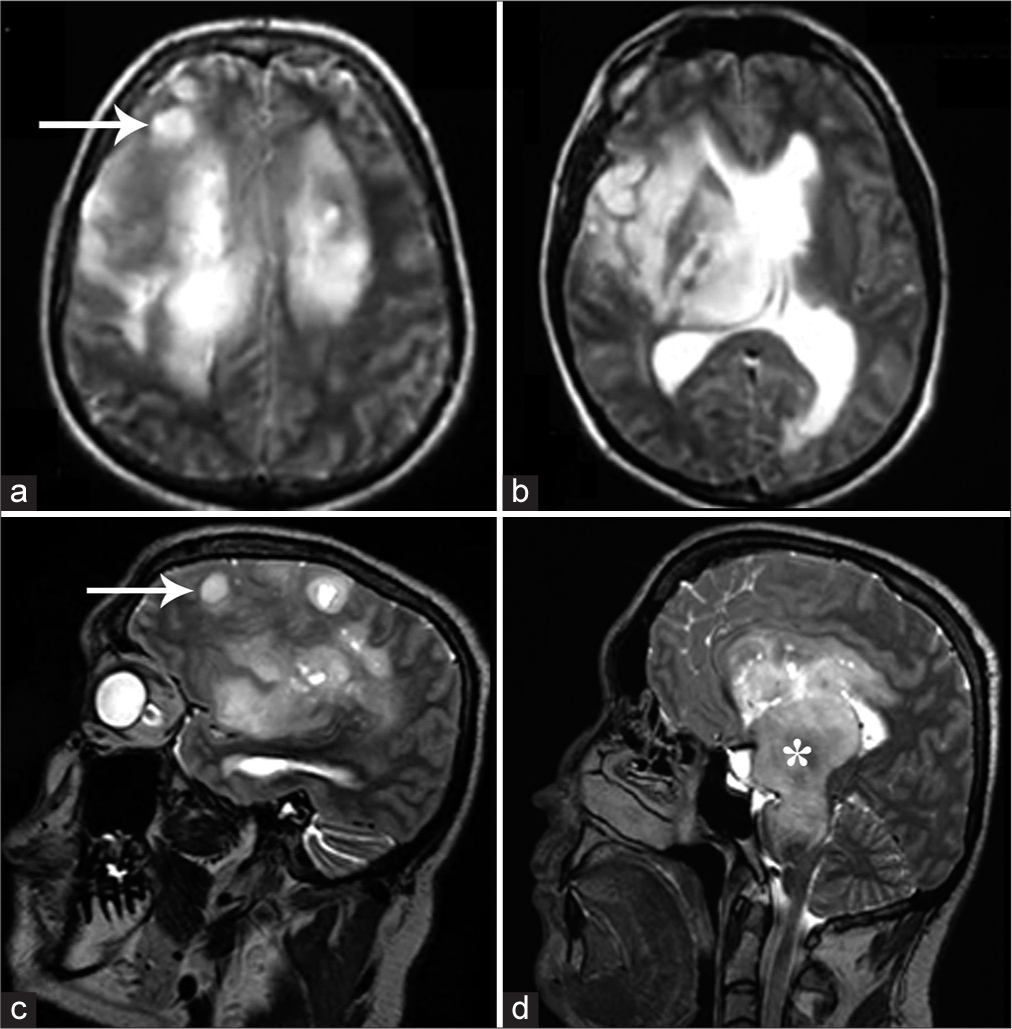
Figure 1:
T2-weighted axial (a and b) and sagittal (c and d) images of magnetic resonance imaging brain showing multifocal T2 hyperintense lesions demonstrating mass effect, involving the thalamus, and extending into the brainstem (white asterisk in D). Some of the lesions were cortical and subcortical in location (white arrows in a and c) and cystic changes were also present.
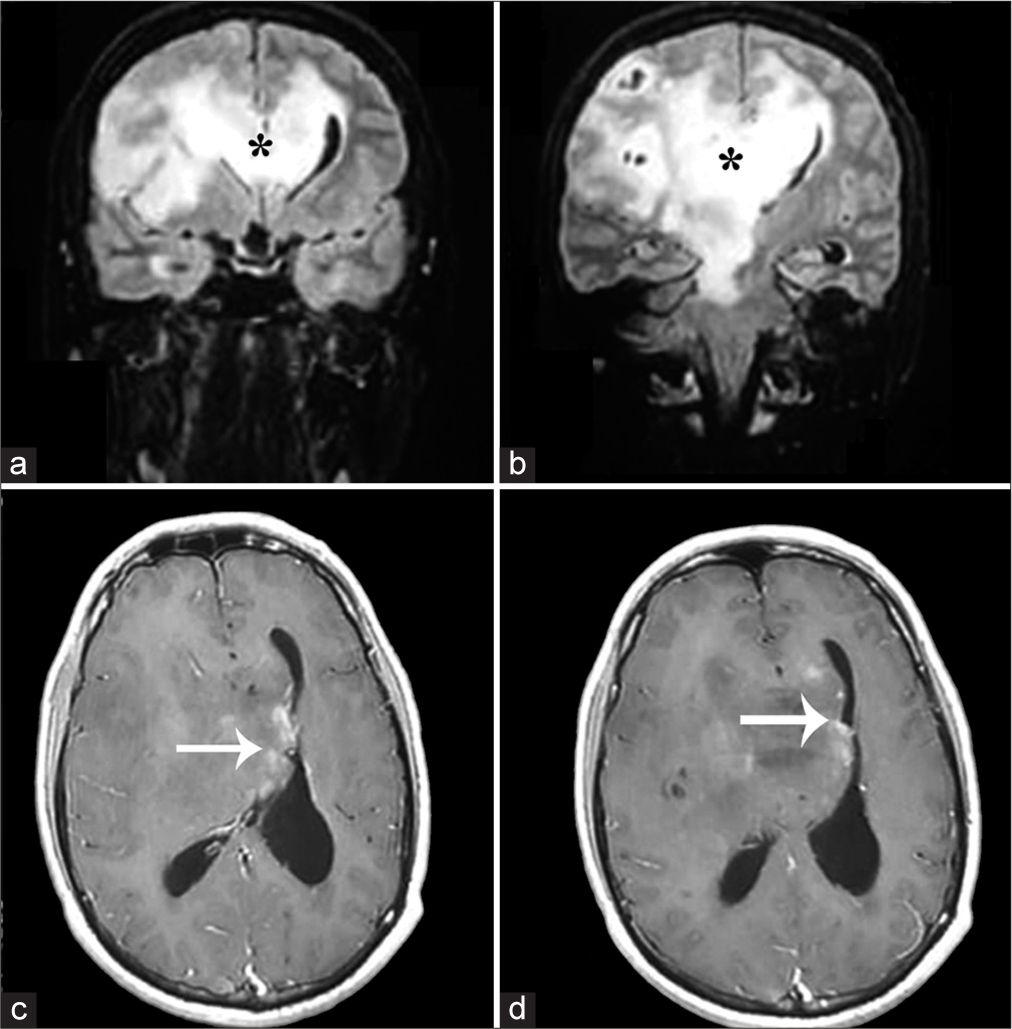
Figure 2:
Coronal FLAIR (a and b) and postcontrast T1 (c and d) images of magnetic resonance imaging brain showing diffusely infiltrative lesion involving the corpus callosum (black asterisk in a and b) and extending to the contralateral side. Patchy lace-like enhancement was seen (white arrows in c and d); however, most of the lesions were largely nonenhancing.
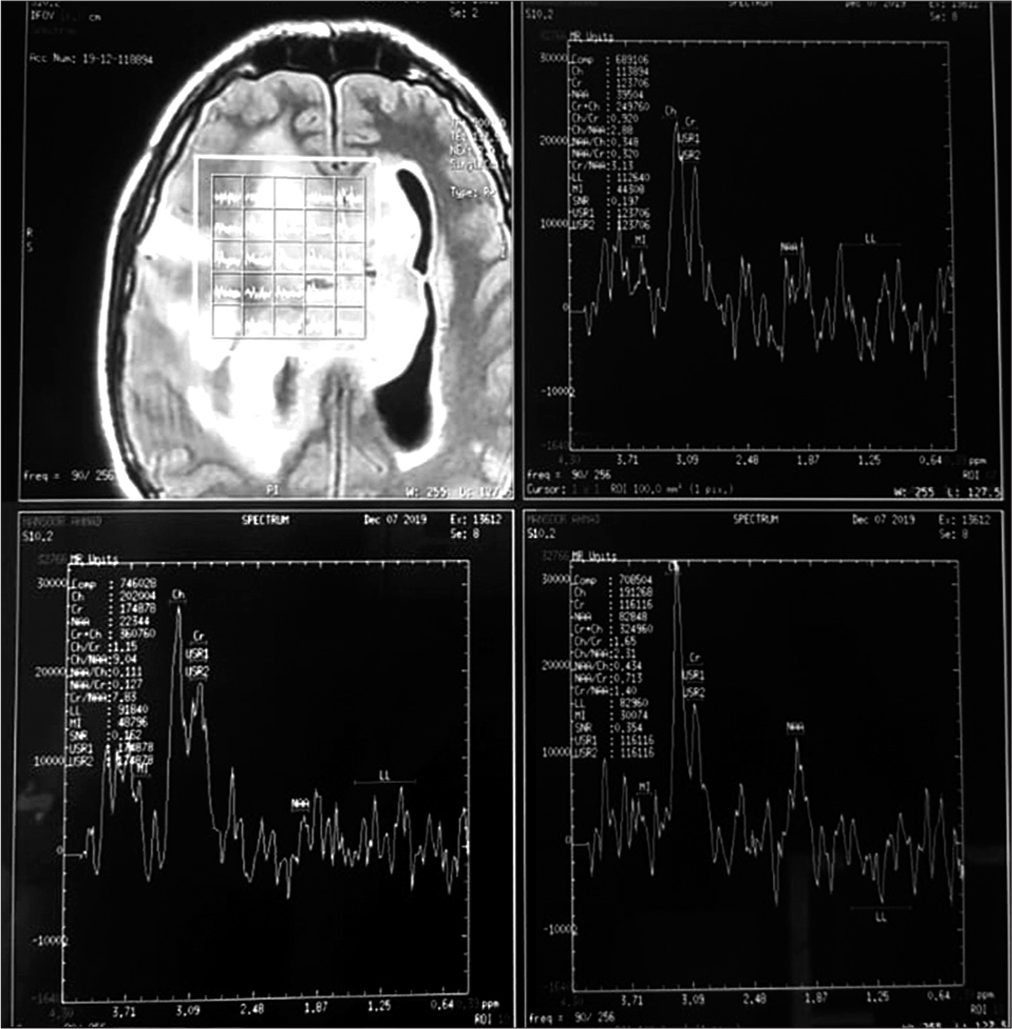
Figure 3:
Magnetic resonance spectroscopy: Frontal lesion showed relative reduction in NAA compared to creatine and an increase in choline compared to creatine. NAA is a marker for normal neurons and axons, creatine is an internal standard and an increase in choline reflects an increase in cellular division. No raised lipid/ lactate peak was noted.
Biopsy was done and microscopy of the lesion revealed a tumor invading glial tissue composed of sheets of round cells with moderate, pale eosinophilic to clear cytoplasm, round to slightly oval vesicular nuclei, and predominantly inconspicuous nucleoli. Characteristic chicken-wire vasculature was present. Occasional scattered mitoses were seen without any endothelial vascular proliferation or necrosis. Tumor cells showed positivity for immunostain glial fibrillary acidic protein, occasional weak positivity for p53, and low Mib-1 (Ki-67) index (1–2%). Immunostain alpha-thalassemia/mental retardation syndrome, X-linked was intact. Based on these combined features, diagnosis of oligodendroglioma, not otherwise specified, WHO Grade II was favored [
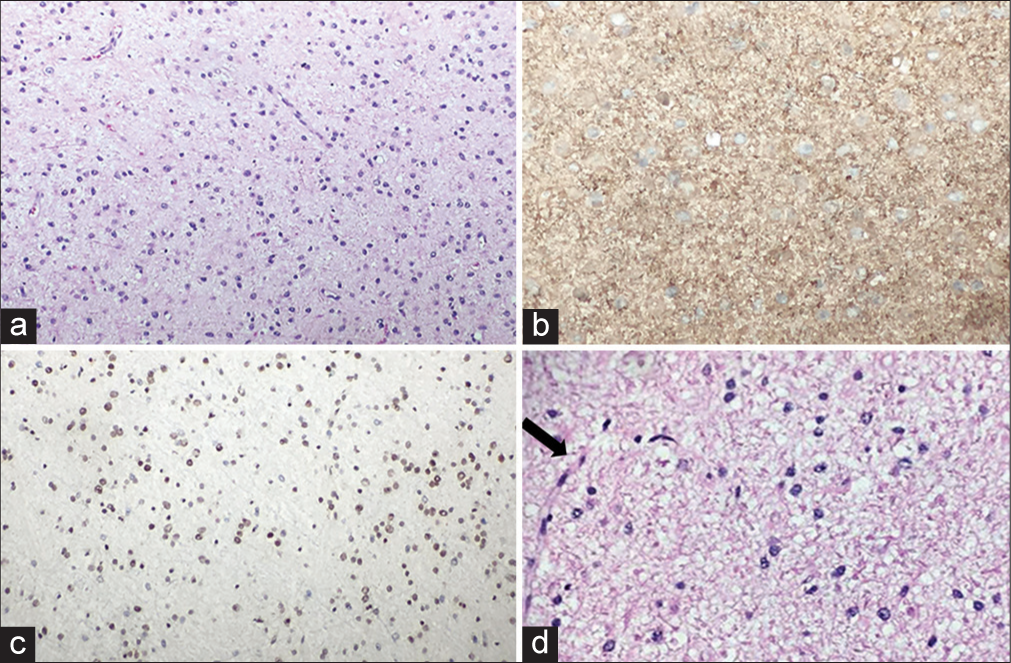
Figure 4:
(a) Sheets of round cells with moderate eosinophilic cytoplasm and scattered thin blood vessels, H&E, 20×. (b) Cytoplasmic GFAP positivity, 20×. (c) Intact alpha-thalassemia/ mental retardation syndrome, X-linked nuclear stain, 20×. (d) High-power view (H&E, 40×) showing characteristic chicken-wire vessel (black arrow).
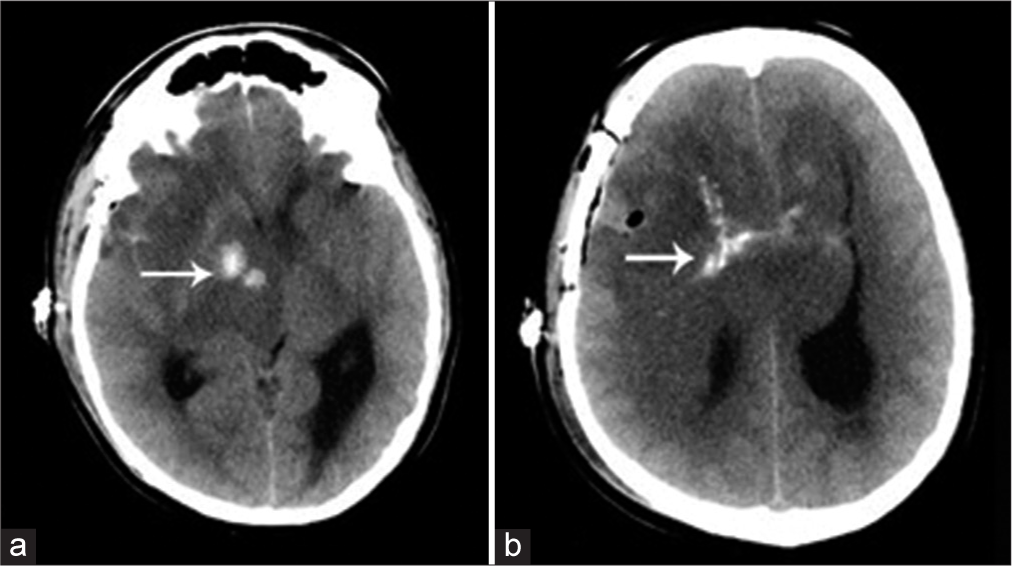
No further surgical treatment was offered due to extensive involvement with a grave patient prognosis. A postbiopsy computed tomography (CT) scan was carried out because the patient had a sudden drop in his Glasgow Coma Scale. The CT scan showed diffuse brain edema and confirmed coarse calcifications within parts of the lesion [
DISCUSSION
Oligodendrogliomas are typically of low density on nonenhanced CT, low signal intensity on T1-weighted, and high signal intensity on T2-weighted MRI. The characteristic imaging features of oligodendrogliomas are the presence of coarse calcification (seen in up to 90% of patients), a cortical-subcortical location, heterogeneously increased signal intensity on T2-weighted sequences, and an indistinct border.[
Occasionally, frontal lobe gliomas may extend through the corpus callosum to produce a “butterfly glioma” pattern; however, this is more common in higher grade glioblastoma. There have been isolated callosal oligodendroglioma reports as well.[
Although uncommon, the occurrence of multiple gliomas has been previously reported. They can be grouped in multicentric and multifocal depending on their mechanism of spread.[
Rare cases of oligodendrocytic gliomatosis cerebri or oligodendrogliomatosis have been reported in the literature, indicating white matter spread through the corpus callosum and brainstem, leading to a detrimental course of neurological status and a poor prognosis.[
CONCLUSION
Multifocal low-grade oligodendrogliomas are uncommon and even though they are initially insidious in their clinical presentation, there is always a concern for potential malignant transformation. MRI and MRS, along with biopsy and histopathological correlation, are needed for a definitive diagnosis. The debate of surgery, neuro-oncological treatments (radiotherapy and chemotherapy) versus imaging surveillance for these types of cases, remains controversial. However, through this case report, we emphasize that radiologists and neurosurgeons should be mindful of such an infiltrative type of pattern of these tumors.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Balko MG, Blisard KS, Samaha FJ. Oligodendroglial gliomatosis cerebri. Hum Pathol. 1992. 23: 706-7
2. Batzdorf U, Malamud N. The problem of multicentric gliomas. J Neurosurg. 1963. 20: 122-36
3. Gandhoke CS, Syal SK, Singh D, Saran RK. Corpus callosal oligodendroglioma: A rare entity. J Case Rep. 2017. 7: 231-4
4. Koeller KK, Rushing EJ. Oligodendroglioma and its variants: Radiologic-pathologic correlation. Radiographics. 2005. 25: 1669-88
5. Monaco EA, Armah HB, Nikiforova MN, Hamilton RL, Engh JA. Grade II oligodendroglioma localized to the corpus callosum. Brain Tumor Pathol. 2011. 28: 305-9
6. Sharifi G, Pajavand AM, Nateghinia S, Meybodi TE, Hasooni H. Glioma migration through the corpus callosum and the brainstem detected by diffusion and magnetic resonance imaging: Initial findings. Front Hum Neurosci. 2020. 13: 472
7. Smits M. Imaging of oligodendroglioma. Br J Radiol. 2016. 89: 20150857
8. Szymaś J, Liebert W. Multifocal oligodendroglioma. A case report. Patol Pol. 1989. 40: 129-34
9. Tancredi A, Mangiola A, Guiducci A, Peciarolo A, Ottaviano P. Oligodendrocytic gliomatosis cerebri. Acta Neurochir (Wien). 2000. 142: 469-72
10. Van den Bent MJ. Anaplastic oligodendroglioma and oligoastrocytoma. Neurol Clin. 2007. 25: 1089-109
11. Van den Bent MJ, Smits M, Kros JM, Chang SM. Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol. 2017. 35: 2394-401
12. Zamponi N, Rychlicki F, Ducati A, Regnicolo L, Salvolini U, Ricciuti RA. Multicentric glioma with unusual clinical presentation. Childs Nerv Syst. 2001. 17: 101-5