- Department of Neurosurgery, Upstate University Hospital, Syracuse, New York, United States .
- Department of Internal Medicine, New York Institute of Technology College of Osteopathic Medicine, Glen Head, New York, United States .
- Department of Pathology, Upstate University Hospital, Syracuse, New York, United States .
Correspondence Address:
George W. Koutsouras, Department of Neurosurgery, Upstate University Hospital, Syracuse, New York, United States.
DOI:10.25259/SNI_985_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: George W. Koutsouras1, Annelle Amsellem2, Timothy Richardson3, Harish Babu1. Multifocal spinal glioblastoma and leptomeningeal carcinomatosis in an elderly male with hydrocephalus and myelopathy. 08-Dec-2021;12:595
How to cite this URL: George W. Koutsouras1, Annelle Amsellem2, Timothy Richardson3, Harish Babu1. Multifocal spinal glioblastoma and leptomeningeal carcinomatosis in an elderly male with hydrocephalus and myelopathy. 08-Dec-2021;12:595. Available from: https://surgicalneurologyint.com/surgicalint-articles/11278/
Abstract
Background: Primary spinal glioblastoma multiforme with multifocal leptomeningeal enhancement is rarely diagnosed or documented. We describe a rare case of multifocal spinal isocitrate dehydrogenase (IDH) wild type glioblastoma with leptomeningeal carcinomatosis in an elderly male presenting with a chronic subdural hematoma, progressive myelopathy, and communicating hydrocephalus.
Case Description: A 77-year-old male with a medical history of an acoustic schwannoma, anterior cranial fossa meningioma, and immune thrombocytopenic purpura presented with right-sided weakness after repeated falls. Magnetic resonance imaging of the brain and spine demonstrated a left-sided subdural hematoma, leptomeningeal enhancement of the brain and skull base, ventricles, and the cranial nerves, and along with florid enhancement of the leptomeninges from the cervicomedullary junction to the cauda equina. Most pertinent was focal thickening of the leptomeninges at T1 and T6 with mass effect on the spinal cord. A T6 laminectomy with excisional biopsy of the lesion was planned and completed. Findings were significant for glioblastoma the World Health Organization Grade IV IDH 1 wild type of the thoracic spinal cord. Subsequently, his mental status declined, and he developed progressive hydrocephalus which required cerebrospinal fluid diversion. Unfortunately, the patient had minimal improvement in his neurological exam and unfortunately died 2 months later.
Conclusion: In a review of the limited literature describing similar cases of primary spinal glioblastoma, the prognosis of this aggressive tumor remains unfavorable, despite aggressive treatment options. The purpose of this report is to increase awareness of this rare condition as a potential differential diagnosis in patients presenting with multifocal invasive spinal lesions.
Keywords: Glioblastoma, Leptomeningeal carcinomatosis, Thoracic myelopathy
INTRODUCTION
Glioblastoma multiforme (GBM) is a World Health Organization (WHO) Grade IV histological malignancy in the central nervous system.[
We describe a rare case of multifocal spinal isocitrate dehydrogenase (IDH) wild type glioblastoma with leptomeningeal carcinomatosis in an elderly male presenting with a chronic subdural hematoma, progressive myelopathy, and communicating hydrocephalus.
CASE REPORT
A 77-year-old male with a medical history significant for acoustic schwannoma, anterior cranial fossa meningioma, and immune thrombocytopenic purpura presented with the right-sided weakness after repeated falls. Non-contrast head computed tomography (CT) scan demonstrated a left-sided subdural hematoma. On examination, he was found awake, but disoriented. He had weakness of the left arm and leg, along with long tract signs that included sustained clonus and hyperreflexia of the bilateral lower extremities. Magnetic resonance imaging (MRI) of the brain demonstrated leptomeningeal enhancement of the brain and skull base, ventricles, and the cranial nerves [
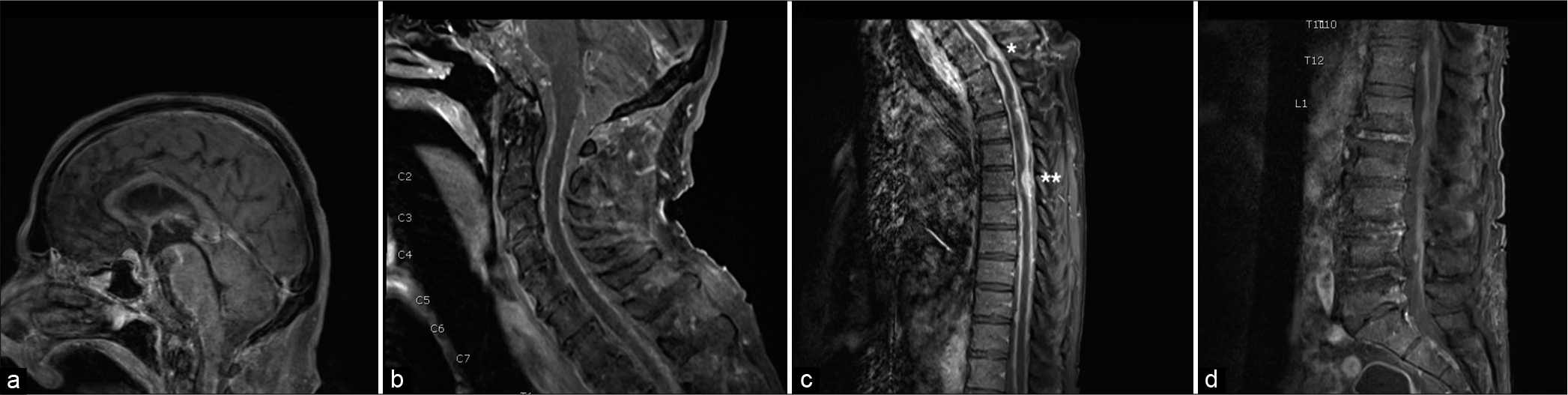
Figure 1:
Magnetic resonance imaging T1 sequences with gadolinium contrast- parasagittal views. (a) Brain - Suprasellar enhancement, leptomeningeal enhancement seen along the ventral brainstem and pineal region. (b) Cervical spine- Diffuse leptomeningeal enhancement seen in the ventral/dorsal spinal cord. (c) Thoracic spine - Dorsally compressive thickened lesions at T1 (single asterick) and T6 (double asterick). (d) Lumbar spine - Diffuse enhancement of the conus medullaris and the cauda equina.
For once having platelets within normal range a month prior, he developed thrombocytopenia with platelets of 40,000 uL. He was initially treated for this thrombocytopenia with intravenous Ig and platelet transfusion with minimal improvement. Once his thrombocytopenia was improved a biopsy was offered to the patient’s family and they wished to proceed.
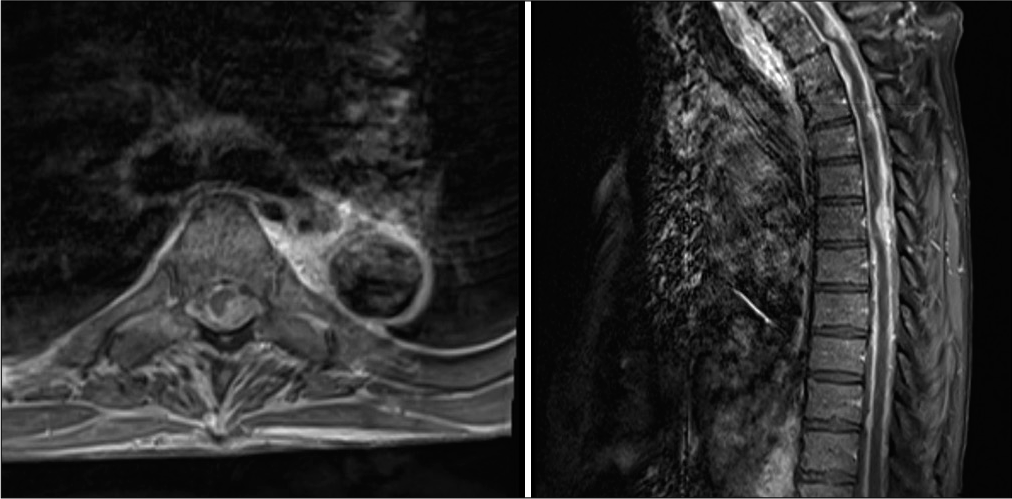
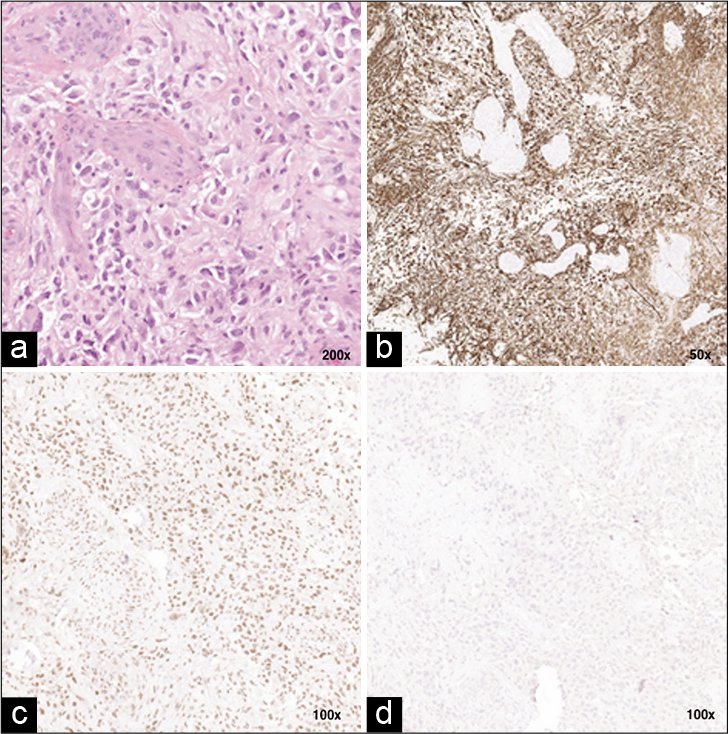
Subsequently, a T6 laminectomy was planned and completed. Intraoperatively, we noted a firm infiltrative lesion of the spinal cord. There was no clear plane separating the tumor and the spinal cord. A subtotal resection was achieved secondary to the infiltrative nature of the lesion. No intraoperative neuromonitoring changes were observed. The pathology report of the biopsied specimens demonstrated hypercellularity with atypical mitotic nuclei and astrocytic features. In addition, there were foci of microvascular proliferation [
Figure 3:
Histopathological stains of T6 lesion. (a) Tumor cells with hypercellularity, atypical mitotic nuclei and astrocytic features. There are foci of microvascular proliferation. Hematoxylin and eosin ×200 (b) Positive stain for Glial Fibrillary acidic protein ×50. (c) Positive for ATRX gene ×100. (d) Tumor cell negative for mutant IDH1 ×100.
Postoperatively, he was fully oriented and following commands in all extremities with more diminished strength and sensation on the right lower extremity compared to the left lower extremity. Over the course of a few days, he developed worsening encephalopathy. A lumbar puncture was completed as part of encephalopathy workup. Elevated opening pressures with an elevated protein level were observed. On placement of a lumbar drain, we identified a meaningful clinical improvement. A ventriculoperitoneal shunt was placed for cerebrospinal fluid diversion. Given the diagnosis with extensive leptomeningeal spread and overall poor neurological function, chemotherapy and radiotherapy were deferred by the oncologists and the family. The patient continued to decline and subsequently passed away 2 months after his surgical diagnosis.
DISCUSSION AND LITERATURE REVIEW
Primary spinal glioblastoma with leptomeningeal disease is a rare diagnosis.[
Spinal GBM most commonly presents in younger patients, as demonstrated by a systematic review where the median age of presentation was 35.5–41 years.[
Our patient demonstrated diffuse cranial and spinal leptomeningeal enhancement with at least one infiltrative tumor at T6. Features commonly associated with spinal GBM include hydrocephalus, increased intracranial pressure, early leptomeningeal infiltration, and malformation of the spinal cord.[
Historically, spinal GBM has been treated with a combination of radiation therapy and temozolomide.[
CONCLUSION
While glioblastomas of the brain are the most common aggressive primary tumor in adults, primary GBM of the spine are exceptionally rare. Spinal glioblastomas are notorious for their nonspecific presentation, aggressive nature, and grim prognosis. Despite their rarity, primary spinal GBM should be considered in the differential diagnosis of scattered spinal lesions.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Thank you to Annette Absellem and Harish Babu, MD for aiding in the completion of this manuscript and Timothy Richardson, MD for his pathology data.
References
1. Abou-El-Ardat K, Seifert M, Becker K, Eisenreich S, Lehmann M, Hackmann K. Comprehensive molecular characterization of multifocal glioblastoma proves its monoclonal origin and reveals novel insights into clonal evolution and heterogeneity of glioblastomas. Neuro Oncol. 2017. 19: 546-57
2. Adams H, Avendano J, Raza SM, Gokaslan ZL, Jallo GI, Quinones-Hinojosa A. Prognostic factors and survival in primary malignant astrocytomas of the spinal cord: A population-based analysis from 1973 to 2007. Spine (Phila Pa 1976). 2012. 37: E727-35
3. Birzu C, Tran S, Bielle F, Touat M, Mokhtari K, Younan N. Leptomeningeal spread in glioblastoma: Diagnostic and therapeutic challenges. Oncologist. 2020. 25: e1763-76
4. Caro-Osorio E, Herrera-Castro JC, Barbosa-Quintana A, Benvenutti-Regato M. Primary spinal cord small-cell glioblastoma: Case report and literature review. World Neurosurg. 2018. 118: 69-70
5. Ciappetta P, Salvati M, Capoccia G, Artico M, Raco A, Fortuna A. Spinal glioblastomas: Report of seven cases and review of the literature. Neurosurgery. 1991. 28: 302-6
6. Delgado BJ, Moosavi L, Rangel E, Stull W, Polineni RD, Chen J. An unusual presentation of spinal giant cell glioblastoma in a 21-year-old female. J Investig Med High Impact Case Rep. 2019. 7:
7. Henson JW. Spinal cord gliomas. Curr Opin Neurol. 2001. 14: 679-82
8. Hernandez-Duran S, Bregy A, Shah AH, Hanft S, Komotar RJ, Manzano GR. Primary spinal cord glioblastoma multiforme treated with temozolomide. J Clin Neurosci. 2015. 22: 1877-82
9. Hsu S, Quattrone M, Ostrom Q, Ryken TC, Sloan AE, Barnholtz-Sloan JS. Incidence patterns for primary malignant spinal cord gliomas: A surveillance, epidemiology, and end results study. J Neurosurg Spine. 2011. 14: 742-7
10. Jeong SM, Chung YG, Lee JB, Shin IY. Intracranial dissemination from spinal cord anaplastic astrocytoma. J Korean Neurosurg Soc. 2010. 47: 68-70
11. Kataria R, Bhasme V, Chopra S, Sinha VD, Singhvi S. Intracranial metastasis of spinal intramedullary anaplastic astrocytoma. Asian J Neurosurg. 2011. 6: 116-8
12. Khalid S, Kelly R, Carlton A, Wu R, Peta A, Melville P. Adult intradural intramedullary astrocytomas: A multicenter analysis. J Spine Surg. 2019. 5: 19-30
13. Konar SK, Bir SC, Maiti TK, Nanda A. A systematic review of overall survival in pediatric primary glioblastoma multiforme of the spinal cord. J Neurosurg Pediatr. 2017. 19: 239-48
14. Lam S, Lin Y, Melkonian S. Analysis of risk factors and survival in pediatric high-grade spinal cord astrocytoma: A population-based study. Pediatr Neurosurg. 2012. 48: 299-305
15. Liu J, Zheng M, Yang W, Lo SL, Huang J. Impact of surgery and radiation therapy on spinal high-grade gliomas: A population-based study. J Neurooncol. 2018. 139: 609-16
16. Luksik AS, Garzon-Muvdi T, Yang W, Huang J, Jallo GI. Pediatric spinal cord astrocytomas: A retrospective study of 348 patients from the SEER database. J Neurosurg Pediatr. 2017. 19: 711-9
17. Moinuddin FM, Alvi MA, Kerezoudis P, Wahood W, Meyer J, Lachance DH. Variation in management of spinal gliobastoma multiforme: Results from a national cancer registry. J Neurooncol. 2019. 141: 441-7
18. Nunna RS, Khalid S, Ryoo JS, Mehta AI. Adult primary high-grade spinal glioma: A nationwide analysis of current trends in treatment and outcomes. J Neurooncol. 2020. 147: 633-41
19. Philippon J. Brain tumors in the elderly. Psychol Neuropsychiatr Vieil. 2004. 2: 29-33
20. Purkayastha A, Sharma N, Sridhar MS, Abhishek D. Intramedullary glioblastoma multiforme of spine with intracranial supratentorial metastasis: Progressive disease with a multifocal picture. Asian J Neurosurg. 2018. 13: 1209-12
21. Sabatino G, Della Pepa GM, Esposito G, Brodbelt A. Glioblastoma multiforme intraxial metastasis to the conus medullaris with primary disease under control: A rare unexpected finding. Br J Neurosurg. 2013. 27: 388-9
22. Scarrow AM, Rajendran P, Welch WC. Glioblastoma multiforme of the conus medullaris. Clin Neurol Neurosurg. 2000. 102: 166-7
23. Shastin D, Mathew RK, Ismail A, Towns G. Cervical spinal glioblastoma multiforme in the elderly. BMJ Case Rep. 2017. 2017:
24. Strik HM, Effenberger O, Schafer O, Risch U, Wickboldt J, Meyermann R. A case of spinal glioblastoma multiforme: Immunohistochemical study and review of the literature. J Neurooncol. 2000. 50: 239-43
25. Tinchon A, Oberndorfer S, Marosi C, Ruda R, Sax C, Calabek B. Malignant spinal cord compression in cerebral glioblastoma multiforme: A multicenter case series and review of the literature. J Neurooncol. 2012. 110: 221-6
26. Urbanska K, Sokolowska J, Szmidt M, Sysa P. Glioblastoma multiforme-an overview. Contemp Oncol (Pozn). 2014. 18: 307-12
27. Yeung YF, Wong GK, Zhu XL, Ma BB, Hk NG, Poon WS. Radiation-induced spinal glioblastoma multiforme. Acta Oncol. 2006. 45: 87-90