- Department of Neurosurgery, Rutgers - Robert Wood Johnson University Hospital, New Brunswick, New Jersey, United States.
DOI:10.25259/SNI_810_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Omar Akel, Bharath Raju, Sumatha Channapatna Suresh, Fareed Jumah, Gaurav Gupta, Anil Nanda. Multiple cerebral hemorrhages in sepsis-disseminated intravascular coagulation versus septic embolism: An image report. 26-Apr-2021;12:185
How to cite this URL: Omar Akel, Bharath Raju, Sumatha Channapatna Suresh, Fareed Jumah, Gaurav Gupta, Anil Nanda. Multiple cerebral hemorrhages in sepsis-disseminated intravascular coagulation versus septic embolism: An image report. 26-Apr-2021;12:185. Available from: https://surgicalneurologyint.com/surgicalint-articles/10758/
Abstract
Background: Septic emboli are commonly attributed to infective endocarditis and can present with a variety of symptoms including altered mental status and focal neurological deficits. Here, we reviewed images of septic emboli with hemorrhagic conversion in a patient with sepsis and a psoas abscess. We aim to show the classical image findings in septic embolism to brain, which is sparsely described in literature and the report differentiates the septic embolism from disseminated intravascular coagulation which can present with almost identical image findings.
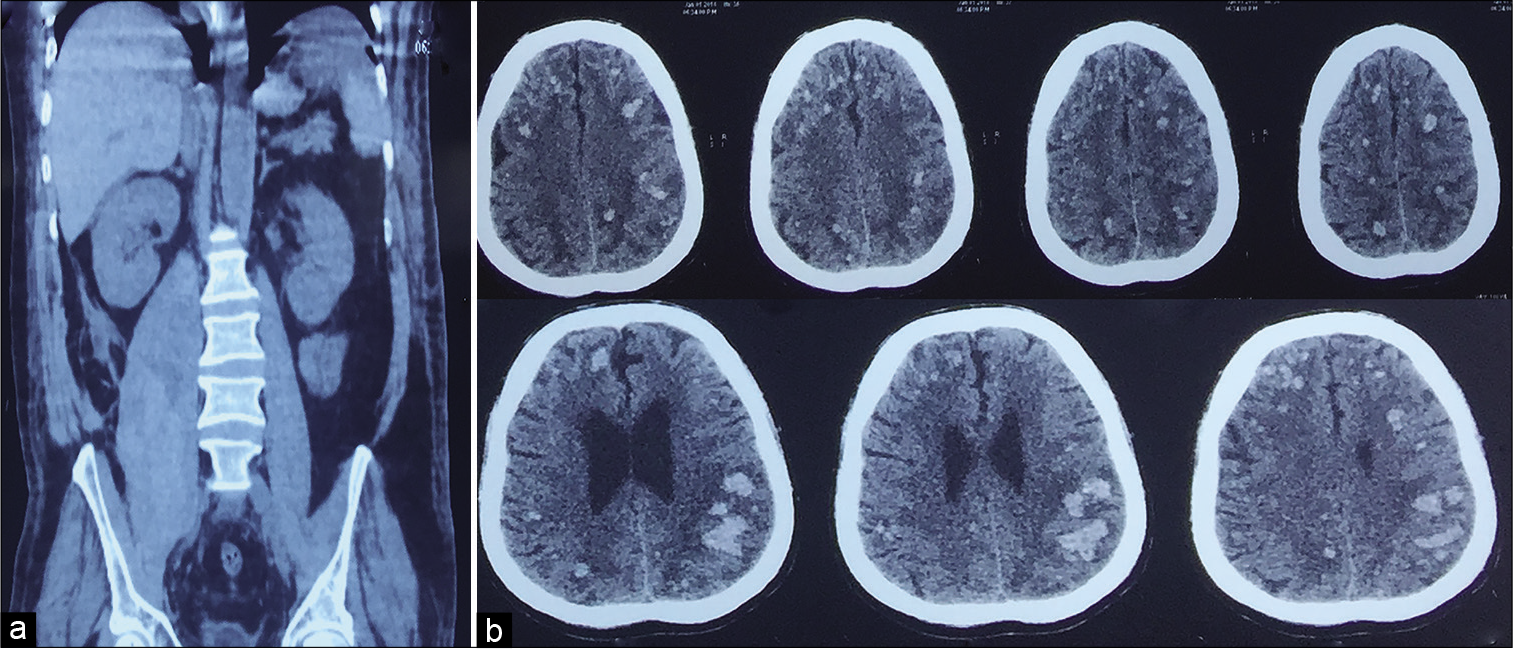
Case Description: A 53-year-old male patient who was operated on for a right inguinal hernia developed a postoperative wound infection 2 weeks after surgery and was started on IV antibiotics. Despite medical management, his infection did not improve, prompting a computed tomography (CT) scan which revealed a psoas abscess. The abscess was drained, and antibiotics continued. A few days later, he developed altered sensorium prompting a head CT which revealed septic emboli and hemorrhage at the gray-white junction. Cultures grew multidrug-resistant Escherichia coli; the patient was treated with IV tigecycline and improved over the following 4 weeks.
Conclusion: In patients with a known ongoing infectious process with hemodynamic stability who develop altered mental status in the setting of a normal coagulation profile, D-dimer, positive blood cultures, and absent signs of multiorgan failure, a diagnosis of septic emboli should be entertained. Although CT can reveal macrobleeds, MRI is more sensitive in confirming cerebral microbleeds. Thus, patients in sepsis with unexplained altered sensorium should undergo an MRI of the brain to rule out septic emboli and microbleeds.
Keywords: Cerebral bleed, Disseminated intravascular coagulation, Psoas abscess, Sepsis, Septic embolism
INTRODUCTION
Septic embolism to the brain from an infective focus is well known. It can present with micro or macro hemorrhages or abscesses involving the cortical grey and white matter junction. The hemorrhagic type of septic embolism can be confused or overlapping with the multiple cerebral hemorrhages of DIC. Here, we compare the imaging findings of septic embolism and DIC with an illustrative case.
CASE REPORT
A 53-year-old male patient who was operated on for a right inguinal hernia subsequently developed a postoperative wound infection 2 weeks after surgery and was started on IV antibiotics. Despite medical management, the patient’s wound discharge did not improve and therefore a computed tomography (CT) scan was conducted which revealed a right psoas abscess. The abscess was partially drained, and he was continued on IV antibiotics. After a few days, the patient demonstrated progressive deterioration of his sensorium. Biochemical and hematological parameters revealed hemoglobin: 14 g/dL, WBC: 18,000 cells/mcL, platelet count: 280,000 cells/mcL, peripheral smear: normocytic and normochromic, neutrophils showing multiple toxic granules, no hemolysis or schistocytes, CRP:14 mg/L, INR: 0.9, D-dimer: normal, and fibrinogen: 2.4 g/suggestive of sepsis. Based on the imaging findings, a diagnosis of septic embolism to the brain was made. Culture sensitivity of the purulent discharge revealed a multidrug-resistant Escherichia coli and was managed with IV tigecycline (100 mg IV, followed by 50 mg IV q12 hourly for 14 days). Over the next 4 weeks, the patient improved dramatically, and repeat imaging showed significant resolution of the hemorrhages.
Imaging findings
CT abdomen with contrast showed an enlarged right psoas muscle with multiple hypodense loculations enhancing peripherally on contrast suggestive of a psoas abscess. CT brain imaging showed diffuse multiple micro- and macro-hemorrhages of varied sizes involving the cortical gray-white matter junction [
Differential diagnoses of multiple cerebral microbleed (CMB)
The detection of CMB alone is nonspecific and can be found incidentally, thus clinical correlation is imperative. The two most common causes are hypertensive small vessel disease and congophilic amyloid angiopathy (CAA). In hypertensive small-vessel disease, CMBs are found in the basal ganglia, thalamus, brainstem, and cerebellum. In contrast, bleed in CAA tends to occur at the corticomedullary junction and can lead to lobar hemorrhage.[
Less common causes include diffuse axonal injury leading to microhemorrhages near the gray-white matter junction. In patients with a known history of malignancy, hemorrhagic micrometastasis from melanoma and renal cell carcinoma can also cause microbleeds. CMBs can also be associated with critical medical illnesses such as sepsis and infective endocarditis; hereditary and idiopathic diseases such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, Fabry disease, Moyamoya disease, and radiation-induced vascular injury.[
DISCUSSION
Definition of micro- and macrobleed
Various cutoffs have been utilized when describing CMB. CMBs have conventionally been defined in the literature as having a maximum of about 5–10 mm, with a minimum of 2 mm.[
Differentiating disseminated intravascular coagulation (DIC) versus septic embolism
A septic embolism is the obstruction of a blood vessel by an infectious nidus, which causes occlusion of the vessel and leads to ischemia and infarction. Septic emboli present a unique challenge because the impact is 2-fold: the ischemic insult caused by the occlusion of the vessel and the infectious/inflammatory insult leading to erosive vasculitis, mycotic aneurysms, or end-organ abscess formation.[
Pathogenesis of bleed in septic embolism and DIC
The pathogenesis of massive bleeding in DIC results from hyperfibrinolysis leading to consumption of clotting factors; without adequate replacement, massive bleeding occurs. Among the four types of DIC, fibrinogen, PT, and platelets are important parameters for diagnosis of the consumptive hemorrhagic type. While no single laboratory finding is diagnostic, the collective trend of the parameters discussed suggests the diagnosis.[
MRI versus CT findings in septic embolism
In evaluating a patient with altered mental status and/or signs and symptoms of neurological insult in sepsis, the imaging modality of choice will depend on the suspected lesion as well as acuity. CT scans have shown a high incidence in detecting cerebral lesions; this incidence, however, is even higher with the use of MRI.[
Septic emboli as previously discussed can lead to ischemic strokes with hemorrhagic transformation, mycotic aneurysms, and focal arteritis. In identifying hemorrhagic lesions, such as lobar hemorrhage and hemorrhagic transformation of an ischemic insult, MRI has showed limited additional value. MRI angiography is also as effective as CT angiography in diagnosing these microbial aneurysms when the diameter is >5 mm.[
MRI use in the setting of sepsis, DIC, and/or septic emboli has shown to be most useful when diagnosing small neurological lesions, such as microinfarcts and CMBs that would otherwise go undetected. One example is the use of susceptibility-weighted MRI in evaluating unexplained neurological and cognitive dysfunction in the context of DIC secondary to sepsis. Neligan et al. reported widespread microhemorrhages in the cortex, subcortex, brainstem, and cerebellum in a septic patient with sickle cell disease presenting in DIC, identifying the etiology of their patient’s neurological deterioration.[
Treatment
Septic emboli to the brain from any source can travel to the cerebral circulation leading to embolic infarcts, cerebral hemorrhages, mycotic aneurysms, and cerebral abscesses.[
Anticoagulation has been a topic of controversy and is avoided with evidence of hemorrhage; to the best of our knowledge, no randomized control trials have been conducted to assess the role of anticoagulation in the setting of septic emboli.[
Embolectomy is an option especially if the embolism is involving major blood vessels like the middle cerebral artery. The benefit of this intervention is subject to controversy and requires evaluation through randomized controlled trials.[
CONCLUSION
In patients with a known ongoing infectious process with hemodynamic stability who develop altered mental status in the setting of a normal coagulation profile, D-dimer, positive blood cultures, and absent signs of multiorgan failure, a diagnosis of septic emboli should be entertained. Although CT can reveal macrobleeds, MRI is more sensitive in confirming CMB. Thus, patients in sepsis with unexplained altered sensorium should undergo an MRI of the brain to rule out septic emboli and microbleeds.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Blitstein MK, Tung GA. MRI of cerebral microhemorrhages. AJR Am J Roentgenol. 2007. 189: 720-5
2. Champey J, Pavese P, Bouvaist H, Kastler A, Krainik A, Francois P. Value of brain MRI in infective endocarditis: A narrative literature review. Eur J Clin Microbiol Infect Dis. 2016. 35: 159-68
3. Cordonnier C, Salman RA, Wardlaw J. Spontaneous brain microbleeds: Systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007. 130: 1988-2003
4. Correa DG, Junior LC, Bahia PR, Gasparetto EL. Intracerebral microbleeds in sepsis: Susceptibility-weighted MR imaging findings. Arq Neuropsiquiatr. 2012. 70: 903-4
5. Ducruet AF, Hickman ZL, Zacharia BE, Narula R, Grobelny BT, Gorski J. Intracranial infectious aneurysms: A comprehensive review. Neurosurg Rev. 2010. 33: 37-46
6. Goulenok T, Klein I, Mazighi M, Messika-Zeitoun D, Alexandra JF, Mourvillier B. Infective endocarditis with symptomatic cerebral complications: Contribution of cerebral magnetic resonance imaging. Cerebrovasc Dis. 2013. 35: 327-36
7. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Salman RA, Warach S. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009. 8: 165-74
8. Haller S, Vernooij MW, Kuijer JP, Larsson EM, Jager HR, Barkhof F. Cerebral microbleeds: Imaging and clinical significance. Radiology. 2018. 287: 11-28
9. Kang G, Yang TK, Choi JH, Heo ST. Effectiveness of mechanical embolectomy for septic embolus in the cerebral artery complicated with infective endocarditis. J Korean Med Sci. 2013. 28: 1244-7
10. Lundy DJ, Trzeciak S. Microcirculatory dysfunction in sepsis. Crit Care Clin. 2009. 25: 721-31
11. Miranda M, Balarini M, Caixeta D, Bouskela E. Microcirculatory dysfunction in sepsis: Pathophysiology, clinical monitoring, and potential therapies. Am J Physiol Heart Circ Physiol. 2016. 311: H24-35
12. Neligan A, Rajakulendran S, Nortley R, Manji H. Extensive cerebral microhemorrhages caused by acute disseminated intravascular coagulation secondary to sepsis. JAMA Neurol. 2014. 71: 510-1
13. Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis. 2010. 2: e2010024
14. Stawicki SP, Firstenberg MS, Lyaker MR, Russell SB, Evans DC, Bergese SD. Septic embolism in the intensive care unit. Int J Crit Illn Inj Sci. 2013. 3: 58-63
15. Wada H, Matsumoto T, Yamashita Y. Diagnosis and treatment of disseminated intravascular coagulation (DIC) according to four DIC guidelines. J Intensive Care. 2014. 2: 15
16. Walker KA, Sampson JB, Skalabrin EJ, Majersik JJ. Clinical characteristics and thrombolytic outcomes of infective endocarditis-associated stroke. Neurohospitalist. 2012. 2: 87-91
17. Zakhari N, Castillo M, Torres C. Unusual cerebral emboli. Neuroimaging Clin N Am. 2016. 26: 147-63