- Department of Neurosurgery King Abdullah Medical City, Makkah, Western Saudi Arabia.
- Department of Interventional Neuroradiology, King Abdullah Medical City, Makkah, Western Saudi Arabia.
Correspondence Address:
Rana Moshref, Department of Neurosurgery, King Abdullah Medical City, Makkah, Western Saudi Arabia.
DOI:10.25259/SNI_944_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sultan Al-Saiari1, Faisal A. Asiri1, Ahmed A. Farag1, Faisal Alghamdi2, Essam Rezk1, Khalid Al Orabi1, Mohammad Ghazi Abdoh1, Jameel Khalid Rasheedi1, Rana Moshref1. Multiple lessons learned from a single case: Complications from pineal germinoma management. 20-Jan-2022;13:29
How to cite this URL: Sultan Al-Saiari1, Faisal A. Asiri1, Ahmed A. Farag1, Faisal Alghamdi2, Essam Rezk1, Khalid Al Orabi1, Mohammad Ghazi Abdoh1, Jameel Khalid Rasheedi1, Rana Moshref1. Multiple lessons learned from a single case: Complications from pineal germinoma management. 20-Jan-2022;13:29. Available from: https://surgicalneurologyint.com/surgicalint-articles/11347/
Abstract
Background: Pineal tumors are uncommon tumors that affect
Case Description: The authors describe an 18-year-old male patient who had a routine, uneventful combined ETV and tumor biopsy, as well as the placement of an EVD. Histopathological examination revealed germinoma. His postoperative course was complicated by IVH after EVD removal, which resulted in clinical vasospasm. Without any prior adjuvant chemoradiation, follow-up MRI of the b rain revealed a significant reduction in the size of the germinoma as well as reconstitution of the patency of the previously obstructed aqueduct of Sylvius.
Conclusion: The take-home message from this case is that in the case of postoperative clinical deterioration in a patient with concurrent IVH and ETV, a high index of suspicion for vasospasm is required, as this may allow a significant amount of blood to pass down to the basal cisterns. Early detection and management of clinical vasospasm are critical for a better neurological outcome. Furthermore, unexpected tumor size changes can occur due to a variety of factors, so recent preoperative MRI of the brain should be obtained in the lead-up to surgery, and postoperative computed tomography should be used sparingly to avoid radiation-related tumor changes.
Keywords: Endoscopic third ventriculostomy, External ventricular drain, Germinoma, Hydrocephalus, Intraventricular hemorrhage, Vasospasm
INTRODUCTION
Pineal tumors are uncommon tumors that affect 1% of adults, with germinoma accounting for up to half of all cases. They cause symptoms of increased intracranial pressure due to obstructive hydrocephalus, as well as endocrine abnormalities and parinaud syndrome.[
Early treatment usually includes a combination of endoscopic third ventriculostomy (ETV) and, if possible, tumor biopsy to relieve the associated hydrocephalus and obtain a tissue diagnosis.
An external ventricular drain (EVD) is usually placed at the end of the procedure as a precautionary measure.[
Cerebral vasospasm is a clinical entity that can occur anywhere from the third to the 21st day after aneurysmal subarachnoid hemorrhage (aSAH). It can cause severe ischemic brain injury, significant morbidity, and a poor outcome if not detected early.[
We present the management of a case of pineal region germinoma that included two unusual events: an unexpected large intraventricular hemorrhage (IVH) following EVD removal in a patient who underwent a successful ETV, resulting in a debilitating clinical vasospasm, and a later significant regression in the size of the pineal region germinoma with no adjuvant chemoradiation given during the patient’s hospital stay.
CASE PRESENTATION
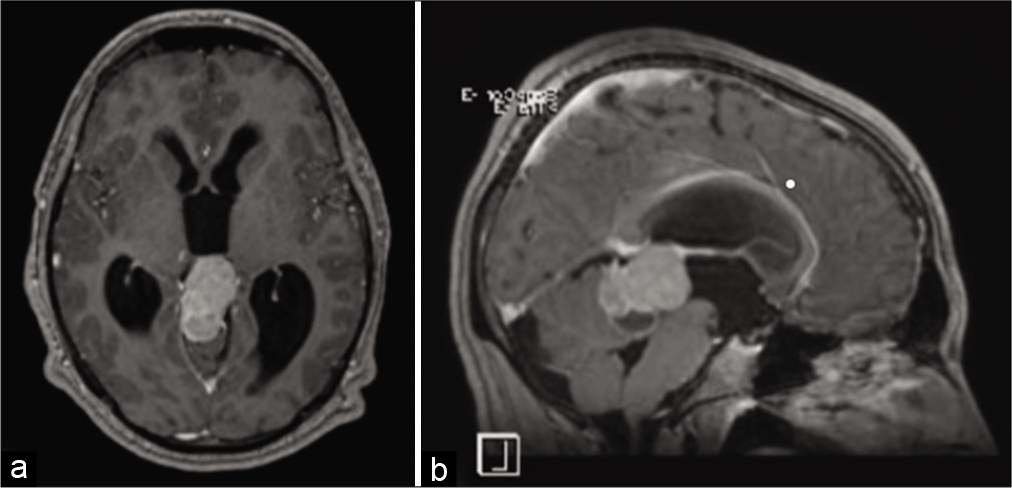
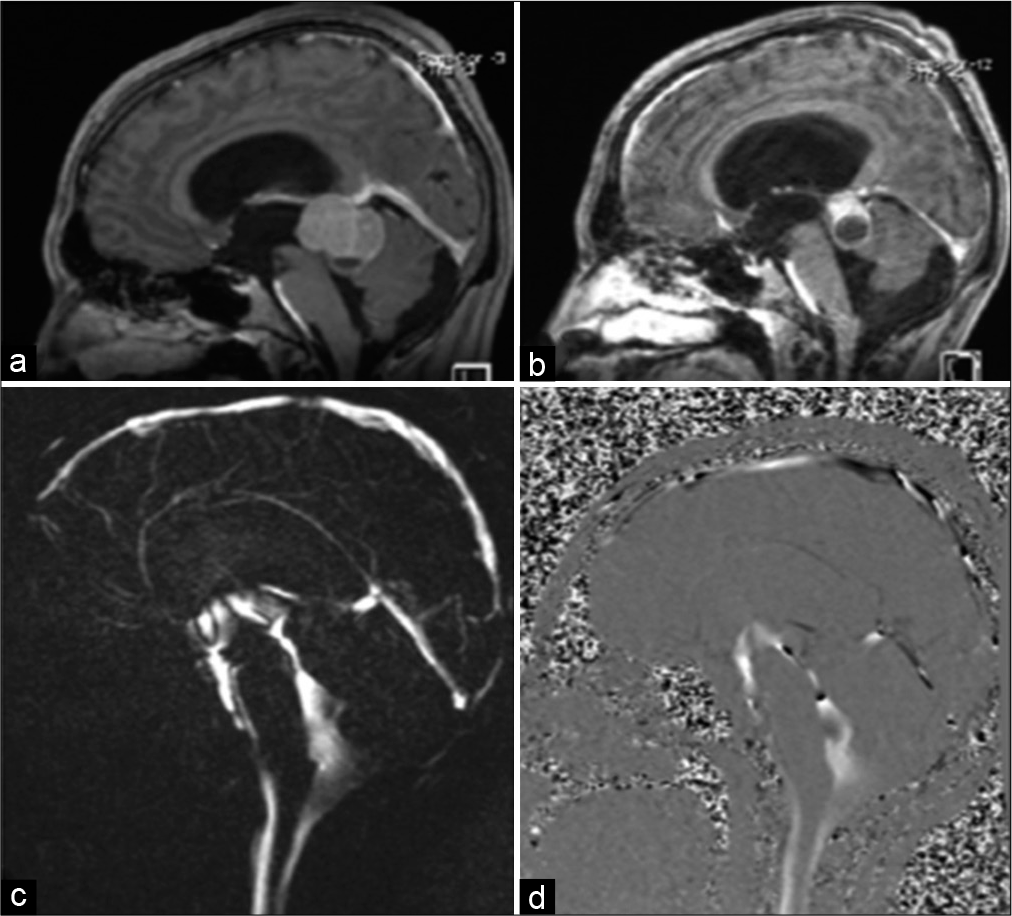
We present the case of an 18-year-old male patient who presented to our emergency department after experiencing progressive headaches for 10 days. The headache was associated with nausea, vomiting, and diplopia. He was conscious and alert at the time of his initial assessment, with a Glasgow Coma Score (GCS) of 15/15. He was ambulating normally but was ataxic on his right side. Fundoscopic examination revealed bilateral papilledema and impaired upward gaze, indicating parinaud syndrome. His laboratory results were within normal ranges. His magnetic resonance imaging (MRI) brain [
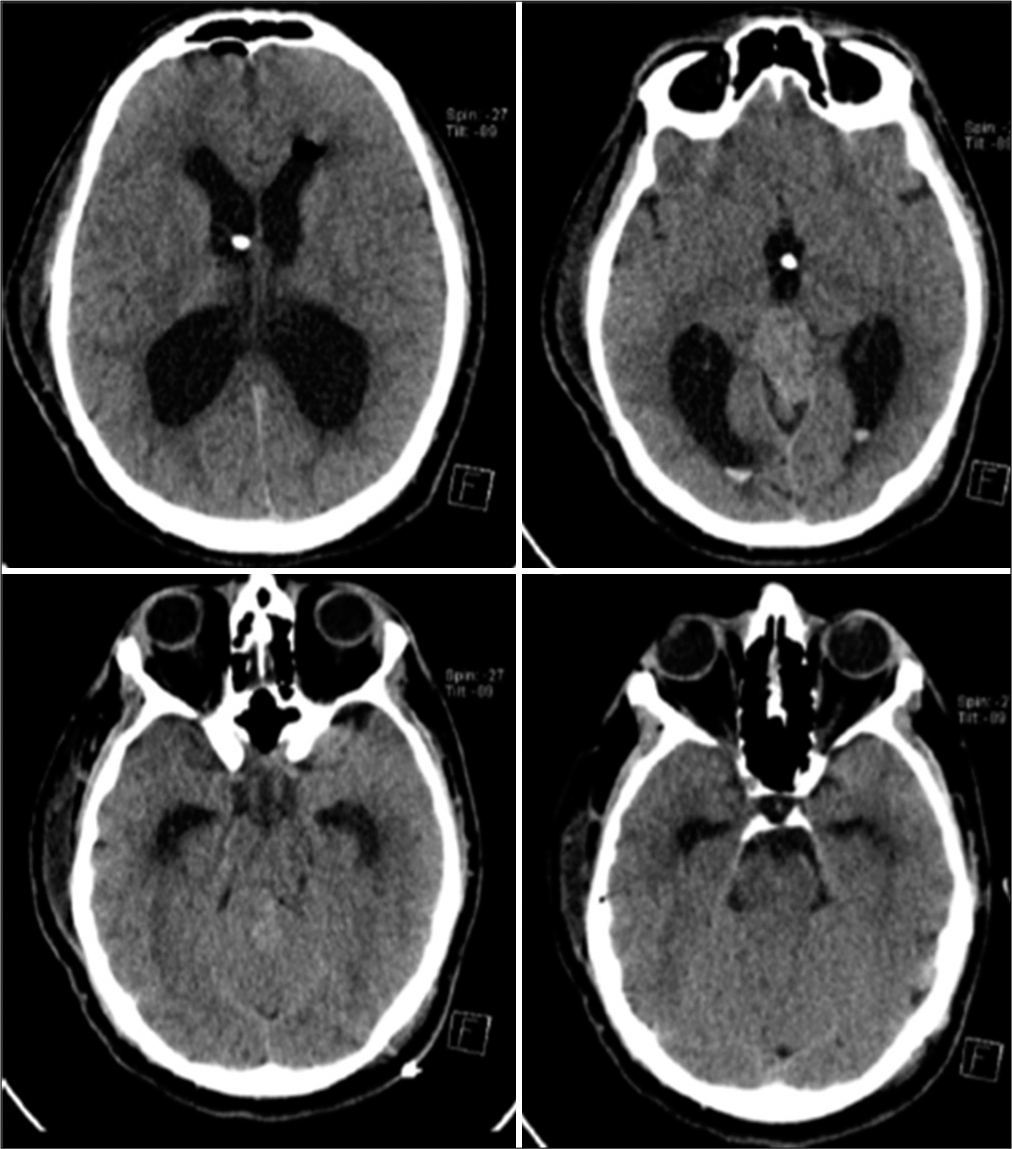
His immediate postoperative noncontrast computed tomography (CT) of the brain [
Figure 2:
Noncontrast CT axial cuts performed day 1 after the ETV and tumor biopsy shows small expected IVH in the occipital horns bilaterally. The tip of the EVD is seen in the third ventricle, and the basal cisterns are free of blood. ETV: Endoscopic third ventriculostomy, EVD: External ventricular drain, IVH: Intraventricular hemorrhage.
From day 1 to day 7 of his ICU stay, the patient was given small dose prophylactic heparin to prevent venous thromboembolism. Our hospital requires all hospitalized surgical adult patients with limited mobility to be placed on thromboprophylaxis if no strong major contraindications are seen from prospective of the treating team’s perspective.
This prophylactic heparin was stopped indefinitely shortly before the EVD was scheduled to be removed on day 7, and the patient was then free to ambulate. The first 7 postoperative days were uneventful; at the time of EVD removal, the cerebrospinal fluid (CSF) in the collecting EVD chamber was clear, with no blood to suggest an IVH.
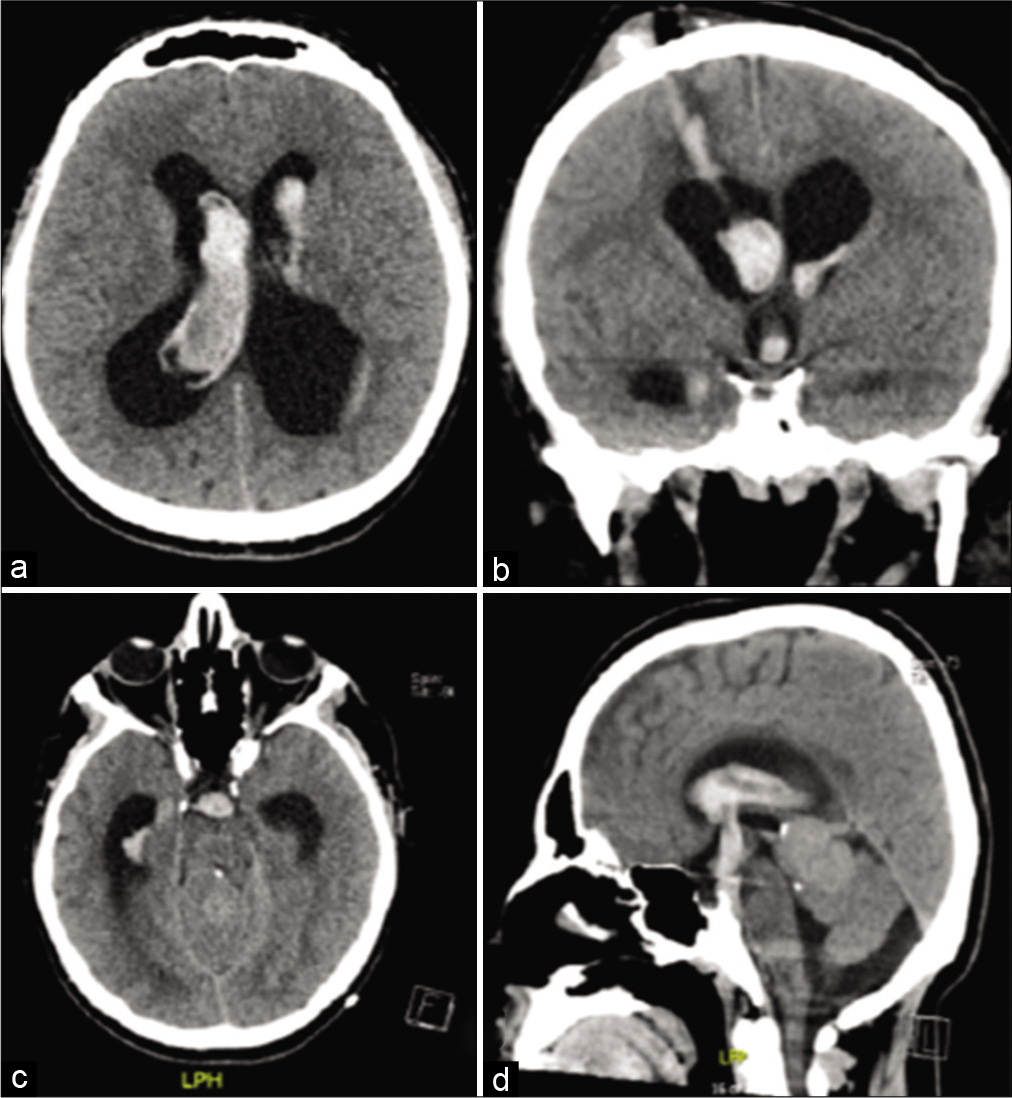
On the 1st post-EVD removal day, the patient complained of generalized neck pain and headache. A repeated CT brain scan revealed a newly developed large IVH [
Figure 3:
Noncontrast axial (a and c), coronal (b), and sagittal (d) CT cuts done day 1 post-EVD removal showing a large IVH. Blood is seen in the track of EVD and descending through the ventriculostomy ostium into the perimesencephalic cistern (b and d). EVD: External ventricular drain, IVH: Intraventricular hemorrhage.
In addition, a follow-up MRI brain performed during the patient’s hospital stay also revealed an unexpected significant interval regression of the size of the germinoma. The Sylvius aqueduct’s patency was spontaneously restored and confirmed by MRI CSF flowmetry [
Figure 6:
Sagittal T1-weighted images postgadolinium enhancement comparing preoperative (a) and postoperative (b) tumor sizes. Marked spontaneous reduction in the size of the germinoma can be seen; previously measured at 4 cm × 3.6 cm × 2.6 cm, and now measuring 2.9 cm × 2.7 cm × 2.1 cm at its maximum anteroposterior, craniocaudal, and transverse dimensions. Magnitude and phase images for CSF flow demonstrate reopening of the aqueduct of Sylvius and the presence of CSF flow in both the aqueduct and the surgically created third ventricular ostium (c and d). CSF: Cerebrospinal fluid.
DISCUSSION
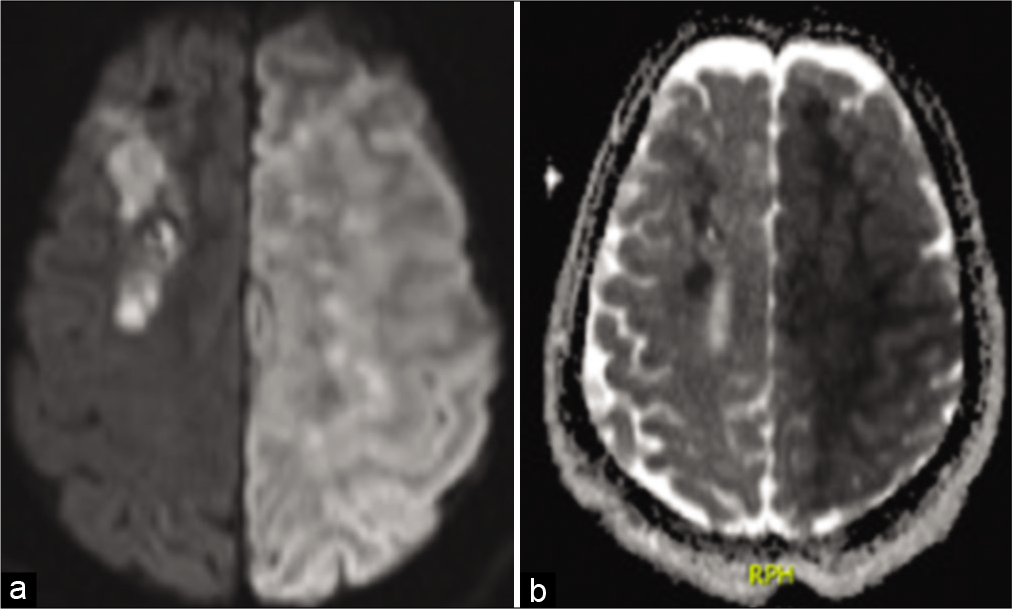
We present a case of a young adult with a pineal region germinoma who received standard treatment, including endoscopic biopsy, ETV, and EVD insertion, but whose course was complicated by IVH, perimesencephalic cistern bleeding, and a devastating ischemic stroke caused by severe bilateral supraclinoid vasospasm. It is uncommon for an isolated IVH to result a full-blown clinically delayed cerebral ischemia. Mensin et al. reviewed 208 papers describing the outcomes of perimesencephalic hemorrhage, radiographic vasospasm was seen in only 9% of them and none of them had clinical DCI.[
The incidence of vasospasm in IVH ranges from 5.6% to 37%.[
Large IVH after the removal of a temporarily EVD is extremely rare. We still do not have enough evidence to determine the cause of our patient’s IVH. Our patient’s coagulation profile was within normal limits. Heparin prophylaxis was stopped before the EVD was scheduled to be removed. There was no blood-tinged CSF in the EVD chamber, indicating intraventricular blood before EVD removal, and there was no resistance when it was removed. We agree Spennato et al. that the probable iatrogenic etiology of such bleeding was blood tracking along the path of catheter from the subgaleal space.[
On the other hand, there was a significant reduction in the size of the germinoma seen on the patient’s MRI, almost by half, in the absence of definitive treatment in the form of adjuvant chemotherapy or radiotherapy. Although spontaneous regression of intracranial germinoma is rare, it has been described in the literature. Schipmann et al. discovered spontaneous regression occurred in 13 cases of pineal region masses, six of which had CNS germinoma, in a review of the literature. Diagnostic radiation therapy, steroid treatment, apoplexy, surgical trauma, and immunological mechanisms were the most frequently mentioned possible causes.[
CONCLUSION
The take-home message from this case is that in the case of postoperative clinical deterioration of a patient with concurrent IVH and ETV, a high index of suspicion for vasospasm is required, as this may allow a significant amount of blood to pass down to the basal cisterns. Early detection and management of clinical vasospasm are important predictors of a better neurological outcome. Furthermore, unexpected tumor size changes can occur due to variety factors; thus, recent preoperative MRI brain images should be obtained in the time leading up to surgery and postoperative CT should be used sparingly to avoid radiation-related tumor changes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank the patient and patient family for agreeing to publish the case report.
References
1. Budohoski KP, Czosnyka M, Smielewski P, Kasprowicz M, Helmy A, Bulters D. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: A prospective observational study. Stroke. 2012. 43: 3230-7
2. Ciurea AV, Palade C, Voinescu D, Nica DA. Subarachnoid hemorrhage and cerebral vasospasm-literature review. J Med Life. 2013. 6: 120-5
3. Favero G, Bonomini F, Rezzani R. Pineal gland tumors: A review. Cancers (Basel). 2021. 13: 1547
4. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980. 6: 1-9
5. Fluss R, Laarakker A, Nakhla J, Brook A, Altschul DJ. Prolonged delayed vasospasm in the setting of nonaneurysmal intraventricular hemorrhage. Surg Neurol Int. 2019. 10: 29
6. Fried HI, Nathan BR, Rowe AS, Zabramski JM, Andaluz N, Bhimraj A. The insertion and management of external ventricular drains: An evidence-based consensus statement: A statement for healthcare professionals from the neurocritical care society. Neurocrit Care. 2016. 24: 61-81
7. Gardner PA, Engh J, Atteberry D, Moossy JJ. Hemorrhage rates after external ventricular drain placement. J Neurosurg. 2009. 110: 1021-5
8. Kiphuth IC, Huttner HB, Breuer L, Engelhorn T, Schwab S, Köhrmann M. Vasospasm in intracerebral hemorrhage with ventricular involvement: A prospective pilot transcranial Doppler sonography study. Cerebrovasc Dis. 2011. 32: 420-5
9. Kobayashi M, Takayama H, Mihara B, Kawase T. Severe vasospasm caused by repeated intraventricular haemorrhage from small arteriovenous malformation. Acta Neurochir (Wien). 2002. 144: 405-6
10. Kothbauer K, Schroth G, Seiler RW, Do DD. Severe symptomatic vasospasm after rupture of an arteriovenous malformation. AJNR Am J Neuroradiol. 1995. 16: 1073-5
11. Maeda K, Kurita H, Nakamura T, Usui M, Tsutsumi K, Morimoto T. Occurrence of severe vasospasm following intraventricular hemorrhage from an arteriovenous malformation. Report of two cases. J Neurosurg. 1997. 87: 436-9
12. Mensing LA, Vergouwen MD, Laban KG, Ruigrok YM, Velthuis BK, Algra A. Perimesencephalic hemorrhage: A review of epidemiology, risk factors, presumed cause, clinical course, and outcome. Stroke. 2018. 49: 1363-70
13. Miller C, Tummala RP. Risk factors for hemorrhage associated with external ventricular drain placement and removal. J Neurosurg. 2017. 126: 289-97
14. Pendharkar AV, Guzman R, Dodd R, Cornfield D, Edwards MS. Successful treatment of severe cerebral vasospasm following hemorrhage of an arteriovenous malformation. Case report. J Neurosurg Pediatr. 2009. 4: 266-9
15. Pople IK, Athanasiou TC, Sandeman DR, Coakham HB. The role of endoscopic biopsy and third ventriculostomy in the management of pineal region tumours. Br J Neurosurg. 2001. 15: 305-11
16. Regula JU, Schill J, Ringleb PA, Sykora M. Cerebral vasospasm and delayed cerebral ischemia in intraventricular hemorrhage. Neurocrit Care. 2014. 20: 460-5
17. Schipmann S, Keurhorst D, Köchling M, Schwake M, Heß K, Sundermann B. Regression of pineal lesions: Spontaneous or iatrogenic? A case report and systematic literature review. World Neurosurg. 2017. 108: 939-47.e1
18. Sobey CG, Faraci FM. Subarachnoid haemorrhage: What happens to the cerebral arteries?. Clin Exp Pharmacol Physiol. 1998. 25: 867-76
19. Spader HS, Doberstein CE, Rao AJ, Jayaraman MV. Central fever as an early predictor of vasospasm in a child with isolated intraventricular hemorrhage. Clin Neurol Neurosurg. 2011. 113: 146-9
20. Spennato P, Mirone G, Aliberti F, Ruggiero C, di Martino G, Cinalli G. Intraventricular hemorrhage following removal of external ventricular drains: Report of 2 pediatric cases. Interdiscip Neurosurg. 2019. 16: 15-7
21. Wilkins RH. Cerebral vasospasm. Crit Rev Neurobiol. 1990. 6: 51-77
22. Yanaka K, Hyodo A, Tsuchida Y, Yoshii Y, Nose T. Symptomatic cerebral vasospasm after intraventricular hemorrhage from ruptured arteriovenous malformation. Surg Neurol. 1992. 38: 63-7